
In skin biology, senescence is a process by which a cell ages and permanently stops dividing but does not die. This is why they are also referred to as "zombie cells". Age-related accumulation of senescent cells is caused by of increased levels of senescence-inducing stressors and/or reduced elimination of senescent cells. Under normal physiological conditions, senescent cells play an important role maintaining cellular homeostasis and inhibiting proliferation of abnormal cells. However, over time, large numbers of zombie cells can build up in the skin and contribute to the overall reduction in skin's regenerative properties, impacting both its beauty and health.
There are 2 forms of cell senescence: Acute senescence: Senescent cells are produced in response to acute stressors to facilitate for example tissue repair, wound healing. They are cleared by our immune system. Chronic senescence: A not programmed process as response to prolonged stress or damage and these senescent cells are not cleared by our immune system, leading to the accumulation of zombie cells impacting our skin health and beauty. It has been suggested that inflammageing is mainly related to senescent cells and their associated SASP (Senescence-Associated Secretory Phenotype) which increase in the body with age and contribute to inflammageing. Senescent cells cause inflammageing and inflammageing causes cell senescence. [1] Senescence can be triggered by a number of stress signals to the cell [1]:
Mechanisms of skin cell senescence:
The presence of senescent cells accelerates the ageing process due to their communication with nearby cells through various molecules: [18]
Fibroblast senescence could be the main driver of the skin ageing. [3] The increased number of senescent fibroblasts results in the production of SASPs rich in pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-8, IL-18, matrix metalloproteinases (MMPs), and a variety of other inflammatory chemokines [2] resulting in the breakdown of collagen, loss of elasticity and wrinkle formation. [3] Autophagy in dermal fibroblasts is essential for maintaining skin balance and managing the ageing process, particularly in response to external stressors like UV radiation and particulate matter (PM), by repairing cellular machineries. [4] Insufficient autophagy leads to an exaggerated skin inflammation triggered by inflammasome activation, resulting in accelerated ageing characteristics. When exposed to UVB (in vitro), skin cell types like fibroblasts and keratinocytes show DNA damage and increased senescence markers, such as increased SASPs. [3] Dermal fibroblasts also release insulin-like growth factor (IGF)-1, essential for epidermal cell proliferation and differentiation. [5] IGF-1 signalling in senescent fibroblasts is significantly decreased [6]. Inhibition of the IGF-1 pathway decreases collagen production in the dermis, causing epidermal thinning. Additionally, mitochondrial dysfunction and increased levels of superoxide anions prompt fibroblast ageing, thereby speeding up the skin ageing process. [5] Fibroblasts isolated from photo-aged skin produce a greater amount of pro-melanogenic growth factors. [14] Ageing-associated pigmentation has also been reported to be driven by (UVA-induced) fibroblast senescence. [15-16] Keratinocyte senescence The epidermis shows less impact of senescent keratinocytes due to their quicker turnover in comparison to fibroblasts. Senescent keratinocytes experience reduced ECM production and cell adhesions [8], along with elevated MMP expression in UV-induced senescence [9], and increased SASP levels, including pro-inflammatory cytokines. [10] Airborn particulate matter (PM2.5) can penetrate a disrupted skin barrier. PM2.5-induced ROS leads to epigenetic modification: reduced DNA methyltransferase, elevated DNA demethylase expression, p16INK4a promotor hypomethylation and therewith accelerated keratinocyte senescence. [11] Keratinocytes are the main type of cells that signal the need for melanogenesis. [12] UVR-induced DNA damage in keratinocytes activates melanogenesis. [13] Melanocyte senescence Senescent melanocytes express markers of inflammageing and dysfunctional telomeres. Senescent melanocyte SASPs induce telomere dysfunction and limit the proliferation of the surrounding cells, hence, senescent melanocytes affect and impair basal keratinocyte proliferation and contribute to epidermal atrophy. [17] STRATEGIES TO COMBAT CELL SENESCENCE PREVENTION Sunscreen: Protection against UV radiation combined with blue light defense (Licochalcone A: powerful anti-oxidant, Nrf2-Activator & increasing Glutathione + Colour pigments) and prevention + repair DNA damage (Glycyrrhetinic Acid) INTERVENTION Senotherapeutics can be classified into three development strategies: [25]
Skin care ingredients: [18]
Of course a healthy life-style and diet (consider also intermittent fasting) will support both your body & skin longevity and beauty Prevention and intervention of skin cell senescence offers a promising approach to improve skin health and beauty. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References
Comments

Like epigenetics and exosomes, neurocosmetics represent a revolutionary approach for skin care incorporating neuroscience principles, leveraging the skin-brain connection to improve skin health and beauty. The term itself is a fusion of the words neuroscience and cosmetics. It differs from psychodermatology which like neurocosmetics connects the interaction between mind and skin, but in a different way. Some describe it as how simple sensory stimulation can improve our overall wellbeing and call it "mood beauty", however this doesn't do it justice as neurocosmetics go beyond mood boosting skincare.
DEFINITION NEUROCOSMETICS Dermatologist Professor Laurent Misery back in 2002 described that neurocosmetics are products which are supposed to modulate the neuro-immuno-cutaneous-system (NICS) function at an epidermal level. Skin cells can produce neuromediators, which are mediators for transmission of information between skin, immune and the nervous system. All skin cells express specific receptors for neuromediators and by binding of the neuromediator to its receptor, modulation of cell properties and skin functions are induced like cell differentiation and proliferation (renewal), pigmentation, etc. Hence, keratinocytes, Langerhans cells, melanocytes, endothelial cells, fibroblasts and the other cells of the skin are modulated and controlled by the nerves and in return skin is able to modulate neuronal activity and growth. [1] SKIN-BRAIN CONNECTION In an article from the International Journal of Novel Research and Developments, the skin-brain connection was described as a psychobiological concept that highlights how emotions, stress, and neurotransmitters impact skin health. Indicating that the skin acts as a neuroimmunoendocrine organ, emphasizing its sensitivity to neural signals and stress responses. [4] CUTANEOUS NERVOUS SYSTEM The skin a sophisticated sensory organ that allows you to interact with your environment through touch and feel. It contains a complex network of nerves that send information about sensations like pressure, pain, itch and temperature from the skin through the spinal cord to the brain [9]. The dynamic interactions between the skin and the nervous system is influenced by factors like stress and inflammation, which can impact skin health and ageing. [7] Nerves in the skin: These nerves are like tiny messengers that tell your brain about what your skin is feeling: pressure, heat or pain. Types of nerve fibers: Some are thick and wrapped in a protective coating, which helps them send messages quickly. Others are thin and slow but are very good at sending messages about pain or temperature changes. [3] Sensory receptors: These receptors can tell if something is touching the skin lightly or if there's a lot of pressure. They can also sense if something is hot, cold, or causing pain. [3] Autonomic nervous system: Part of the cutaneous nervous system helps control things that happen in the skin automatically, like sweating to regulate body temperature. [8] Nerve cells: There are about 20 different types of neurons in our skin. [10] The contribution of epidermal keratinocytes to NICS [3]
CUTANEOUS NEURO-AGEING Neuro-ageing is defined as the changes in the nervous system which cause continuous neurodegeneration due to oxidative stress, neuroinflammation or impaired neuromodulation. As skin ages, Aβ-toxin (increased by oxidative stress) accumulates at the nerve endings innervating the tissue, causing disrupted cellular communication, particularly affecting fibroblasts’ ability to produce collagen and extracellular matrix. On top there is a decrease of nerve growth factor (NGF) production, important for the development and maintenance of nerve cells. Different factors can lead to a drop in NGF production, resulting in malfunctioning keratinocytes and reduced lipolytic activity of adipocytes, visibly impacting skin hydration and firmness. [6] Skin nerve fibres are significantly reduced in number following UV irradiation and in ageing skin [5] and therefore neuro-protectors or targetting neurodegeneration can reduce stress manifestations and promote healthy cellular communication for optimal skin function. [3] Although not much is known regarding skin specific or topical neuroprotectors (most research was focussed on the brain), probably potent anti-oxidants, by significantly reducing oxidative stress from UV and blue light and anti-inflammatory ingredients may inhibit skin neuro-ageing and can be neuroprotective especially when combined with sunscreen and strengthening of the skin barrier. NEUROCOSMETIC VARIETY OF ACTIONS
THE FUTURE OF NEUROCOSMETICS The neurocosmetics market is booming, with a projected value of USD 2.69 billion by 2030. [11] The future of neurocosmetics holds promise for innovative ingredients and concepts that harness new neuroscientific insights to revolutionize skin care and sunscreen formulations, to cater to both physical and emotional aspects of skin health and beauty. Take care! Anne-Marie References

One of the people I follow ever since I started to work on skin epigenetics back in 2017 and longevity is Harvard professor David Sinclair. He is best known for his work on understanding why we age and how to slow its effects. He was talking about hormesis, a phenomenon where exposure to low doses of stressors induces beneficial effects. A hormetic (cellular defense) response can modulate ageing processes by activating genes related to maintenance and repair pathways through mild stress exposure in our body and skin, leading to enhanced longevity (thus anti-ageing) and health. [1 - 2]
Originating from the early 2000s, the concept of hormesis has evolved to evidenced based dermatological applications. [3] Various factors, including environmental stressors, lifestyle choices, and genetic predispositions, can influence the hormetic responses in skin cells. Understanding these influences is essential for optimizing skin health and beauty through hormetic pathways. Many terms are used for hormetic responses in the scientific literature, including the Arndt-Schulz Law, biphasic dose response, U-shaped dose response, preconditioning/adaptive response, overcompensation responses, rebound effect, repeat bout effect, steeling effect, among others. [4] Ageing is an emergent, epigenetic and a meta-phenomenon, not controlled by a single mechanism. Cellular damage has three primary sources: [3]
Effective homeodynamic space or buffering capacity (body's ability to maintain stability or balance in changing conditions) is characterized by:
Stress response is a reaction to physical, chemical, or biological factors (stressors) aimed at counteracting, adapting, and surviving, is a critical component of the homeodynamic space. There are seven main cellular stress response pathways:
Hormetins can be categorized into three types:
Being aware of the phenomenon of hormesis can result in discovering the usefulness of new compounds, or synergistic effects of combining hormetic treatments which otherwise may have been rejected due to their effects of stress induction. What is bad for us in excess, can be beneficial in moderation, or (quote): "What doesn't kill you makes you stronger". [6]. The future of hormesis in dermatology holds great promise for innovative interventions, advanced hormetic technologies or personalized skin care regimens. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie Read more: The impact of senescent zombie cells on skin ageing The role of heat shock proteins in skin rejuvenation Neurocosmetics, the skin-brain connection & neuro-ageing The role of the lymphatic system in ageing skin The power of light and photo-biomodulation Bio-stimulators Skin glycation Exosomes References

If you've scrolled through Instagram, you may have caught a glimpse of dermatologists raving about LED masks emitting red light, the secret, evidenced based weapon behind skin rejuvenation known as photo biomodulation. It uses low-powered light within the red to near-infrared range (wavelengths from 632 to 1064 nm) to induce a biological reaction aka stimulate cellular processes. The wonders of red light, also known as LLLT (low-level laser therapy), PBM (red light photo-biomodulation), or PBMT (photo-biomodulating therapy), extend far beyond non-invasive skin rejuvenation. I am not a fan of devices for home use, mostly because of lacking safety and/or efficacy, PBM definitely earned it's prominent spot in my skincare routine.
A summary of the benefts of red light with and without near infrared light for skin Numerous studies have demonstrated the effectiveness of red and infrared light therapy for skin rejuvenation. A combination of red light and near IR light has proven to stimulate the production of collagen (I & III) plus elastin production (Li WH et al Int J Cosmet Sci 2021), enhance mitochondrial ATP production, cell signaling, growth factor synthesis, rebalance ROS (reactive oxidative species) and reduce inflammation. Stem cells can be activated allowing tissue repair and healing. Wrinkle and scar reduction was observed and it can reduce UV damage both as treatment and prophylactic measure. In pigmentary disorders such as vitiligo, it can increase pigmentation by melanocyte proliferation and reduce depigmentation by inhibiting autoimmunity (Pinar Avci et al. Semin Cutan Med Surg. 2013 & Mitchell J Winkie et al. Review Photodermatol Photoimmunol Photomed A focused review of visible light therapies for vitiligo 2024). It has the potential to activate both keratinocytes (epidermis) and fibroblasts (epidermal junction and dermis). With consistent use, you can expect a reduction of lines and wrinkles, improvement of skin tone and texture. PBMT (when done effective and safe) will compliment both your skin rejuvenating and regenerating at home skincare regimen and in-office procedures or even post-surgical skin recovery. ATP ATP (adenosine triphosphate) is the primary source of energy for cellular processes and plays a crucial role in various biological functions. When red light with specific wavelengths (630 nm to 638 nm and 810 nm) is absorbed by the skin cells, it stimulates the mitochondria, which are the powerhouses of the cells responsible for ATP synthesis. This increase in ATP production is providing cells with more energy to carry out their functions effectively and has several beneficial effects on the skin like boosting cellular metabolism, promoting more efficient nutrient uptake and waste removal. The increased ATP levels facilitate collagen synthesis by fibroblasts, a vital component for skin structure, elasticity and firmness and reduction of lines and wrinkles.. ATP aids in the repair and regeneration of damaged skin cells. It accelerates the healing process, making it beneficial for wound healing, post-surgical recovery, and addressing skin issues such as acne scars. ROS (Reactive Oxidative Species) By modulating ROS levels, red light therapy helps reduce oxidative stress and its detrimental effects on the skin. ROS are highly reactive molecules that are naturally produced by cells as byproducts of metabolic processes. While low levels of ROS play important roles in cellular signaling and immune responses, excessive ROS can lead to oxidative stress and damage to cells and tissues. Restoring the balance of ROS result in improved skin health, reduced inflammation, and enhanced skin rejuvenation. Red light therapy has been shown to modulate reactive oxidative species (ROS) levels in the skin by promoting antioxidant defense mechanisms and reducing oxidative stress:
The difference between LLLT and PBM LLLT refers specifically to the use of lasers, which produce coherent, focussed and an intense beam of monochromatic light, while PBM has a broader range of light sources, may include laser as well as light-emitting diodes (LEDs) and other non-laser devices. LEDs are often used in PBM because they are cost effective, versatile and have the ability to cover large treatment areas. LLT uses higher power densities with more energy and has a shorter treatment duration in comparison to PBM to achieve desired therapeutic effects. While there are similarities in terms of mode of action", there is a difference of light source, treatment application and parameters. Based on consensus, PBM and PBMT are considered the correct way to describe this photonic specialty for therapeutic applications. In this post I will focus on PBM and specifically LEDs. LED masks and LED panels LED masks specifically produced by the brand Omnilux (FDA cleared) are currently very popular for very good reasons; they are safe and effective when the LEDs emit the right wavelengths and used in the recommended frequency. Omnilux combines 2 therapeutically effective and complimentary wavelengths: 633nm and near-infrared 830 nm. Both wavelengths (more precise 630nm + 850nm) I would recommend to minimally look for in any red LED device, which will disqualify most LED masks and panels in the market! I've include some (not affiliated) links to devices below. Both masks and panels can be effective, however most panels are stronger in comparison to masks 60 mW/cm² vs mW/cm²), hence have the benefit of a shorter treatment time to get a similar result. Intensity and power of red light therapy devices are typically measured in terms of irradiance (measured in milliwatts per square centimeter, mW/cm²) and radiant flux (measured in watts, W), which quantify the amount of light energy emitted by the device. Wearing a mask during a hot summer or in a warmer climate will make you sweat and depending on the materials of the mask and straps, they may be very uncomfortable to wear. Panels have the benefit that they give a more even distribution of emitted light as masks are worn on the face and thus the LED bulbs are pushed on a small skin surface area, panels can cover a larger area (depending on their size) and are more versatile in use, as area's like neck, décolletage, or knees are easier to treat with a panel. With a mask you may be more mobile, although I would not recommend walking around while using the mask. My personal preference would be a panel for the reasons mentioned before and panels are more suitable (more hygienic) for family sharing. My son can use it after an intense workout to speed up his recovery and I like to use it for purposes beyond photo-biomodulation or skin rejuvenation, for example to improve my sleep. With a panel I get more "bang for my buck". 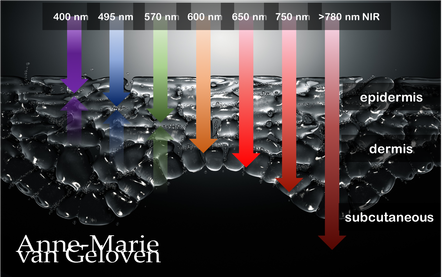
Red light and NIR (Near Infra Red light) have the ability to penetrate varying depths of the skin, resulting in distinct benefits, thus combinations of wavelengths will provide complementary effects.
630 nm Wavelength This wavelength is often used for its skin rejuvenation benefits. It has a relatively shallow penetration depth and is absorbed closer to the surface of the skin primarily affecting the epidermis. 630nm light is associated with increased circulation, reduce inflammation, improved skin tone & texture, aiding in the delivery of nutrients and oxygen to skin cells, and stimulating the production of collagen, leading to improved skin elasticity and a reduction of the appearance of fine lines & wrinkles. 660 nm Wavelength At 660nm, red light can penetrate a little deeper into the skin, reaching the dermis. It is known for its ability to stimulate collagen production, enhance cellular metabolism, and promote anti-inflammatory effects, helping to reduce redness and inflammageing. It also promotes wound healing, making it beneficial for post-surgical or post-trauma skin recovery. 810 nm Wavelength Improve healing & recovery & accelerate wound healing. 830 nm Wavelength Accelerate healing, reduce infection, improve aesthetic outcome following plastic surgery, increase endorfines (mood-enhancing), improve bone repair and growth. 850 nm Wavelength Improve general inflammation body, enhance muscle recovery, improve wound healing, reduced fine lines, wrinkles and hyperpigmentation. Always consult a qualified healthcare professional or dermatologist to determine if and what the most suitable red light therapy approach is for your particular skin condition and rejuvenation goals. Take care! References: Hamblin, Michael R. "Mechanisms and applications of the anti-inflammatory effects of photobiomodulation." AIMS biophysics 4.3 (2017): 337-361. Barolet, Daniel. Regulation of Skin Collagen Metabolism In Vitro Using a Pulsed 660 nm LED Light Source: Clinical Correlation with a Single-Blinded August 2009Journal of Investigative Dermatology 129(12):2751-9 Wunsch A, Matuschka K. (2014). A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Journal of Cosmetic and Laser Therapy, 16(5), 232-237. Avci P, et al. (2013). Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Seminars in Cutaneous Medicine and Surgery, 32(1), 41-52. Links to some devices which combine 630 nm and 850 nm: FDA-approved devices ensure safety and regulatory compliance, however the panels are more powerful: Omnilux(tm) Mask (FDA clearance) Very affordable panel (no FDA clearance) Affordable panel (no FDA clearance) 5/11/2023 Comments The right amount of skin care
Using the right amount of a skin care product is as important as picking the right product(s). If you don't apply enough of the product or for a too short duration, you will not get the optimal result. This is particularly crucial when using sunscreen to reach the sufficient SPF level and protection. According to a study published in the Journal of the American Academy of Dermatology by Andreas Storm MD et al. 95% of patients with a topical treatment under-dose, hence do not use enough cream.
If there is a specific user manual mentioning the dosage, or you got a prescription, follow their recommended instructions. If the product came without specific dosage instructions, there is a general rule of thumb. The recommended amount of product to apply varies, depending on the product type. THE 2 FINGERS RULE FOR SUNSCREEN For sunscreen you need 1/2 teaspoon for the face or enough to cover the bottom of a shot glass and a full shot glass for the body, which should add up to 2mg per cm2. Another method is using the "rule of nines, which is used for burns. The body areas are divided into 11 area's, each representing 9% of the total. Sunscreen can be applied to each of these areas at a dose of 2 mg/cm2 (regardless phototype) if two strips of sunscreen are squeezed out on to both the index and middle fingers from the palmar crease to the fingertips, thus 2 fingers. (1) The body areas are: 1 Head, neck, and face 2 Left arm 3 Right arm 4 Upper back 5 Lower back 6 Upper front torso 7 Lower front torso 8 Left upper leg and thigh 9 Right upper leg and thigh 10 Left lower leg and foot 11 Right lower leg and foot FINGERTIP UNITS For the use of other topical products there is a guidance created called Finger Tip Units or FTU's by CC Long and AY Finlay. It is a way of measuring the amount of product squeezed out of a tube with a 5mm diameter nozzle and applied from the distal skin-crease (the crease closed to the fingertip) to the tip of the index finger. The FTU concept has been used as a central part of an education programme for parents of children with atopic eczema, has been advocated to reduce the variation in usage of topical steroids and to encourage adherence to therapy. For a serum, you may need less as they are lightweight products which should be fully "absorbed" without residue. If the skin still feels sticky after 1 minute, you probably applied too much product. A guidance would be a pea size dot on forehead, right cheek, and left cheek, which is similar to the recommended amount of retinoids (Vitamin A). However, unlike Vitamin A, using too much serum usually isn't harmful for the skin, but increases the risk of "pilling". 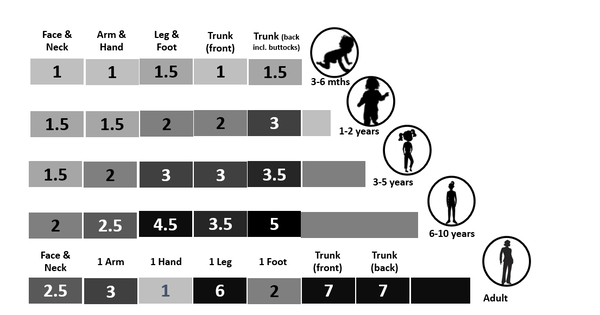
The precise number of FTU's required:
One FTU covers 286 cm2, more specifically in males and 312 cm2 in females 257 cm2. The quantity of cream in a fingertip unit varies: Adult male: 1 fingertip unit provides 0.5 g Adult female: 1 fingertip unit provides 0.4 g . Keep in mind this is a general guideline and the amount of product needed or results may vary also depending on skin type, concerns and the products particular attributes. Take care (in the right amount and duration) References: 1. BMJ. 2002 Jun 22; 324(7352): 1526.Simple dosage guide for suncreams will help users Steve Taylor et al. Illustration Tinea incognito with unjustified use of potent Topical Corticosteroids: a case series July 2017 International Journal of Basic & Clinical Pharmacology 6(8):2087 Haiya Sheth et al. 5/6/2023 Comments The impact of humidity on skin
Something I am asked quite regularly is if low humidity can dry out the skin. The answer is yes it can. However, it really depends on your skin. There are 4 skin types: normal, dry, combination and oily. According to the (AAD American Academy of Dermatology) sensitive skin is a skin type too, however some would say all skin is sensitive and I somewhat agree. Dehydrated skin is not a skin type, but a (temporary) condition. Your skin type is pretty much set for life and not changing with the seasons. There is an exception as normal, oily or combination skin may become dry(er) post-menopause. The environment, including temperatures and humidity impact our skin significantly.
Humidity Humidity is the amount of water vapor in the air. If there is a lot of water vapor in the air, the humidity will be high. A relative humidity of 70 percent means the air is at 70 percent of its water-holding capacity for the present temperature. Cold air cannot hold as much water vapor as warm air. Thus, as temperature falls, with no change in the amount of water in the air, the relative humidity rises. HIGH HUMIDITY A study showed that in a dry environment the skin hydration decreases but the amount of sebum increases to compensate for skin dryness. (1) High humidity can be beneficial if you have dry skin, however can cause problems if you have oily or combination skin. Low levels of humidity can negatively affect your skin, even when your skin is oily. In general high humidity levels has some benefits. Hydration The increased levels of moisture in the air in high humidity decrease trans-epidermal-water loss of water evaporation from the skin. Hence, your skin is able to maintain it's hydration levels. Increased cell regeneration A moisture-rich climate can also promote skin cell turnover and desquamation (the shedding of dead skin cells). Skin’s regeneration process is increased when your skin is well hydrated. The shedding of dead skin cells is the last step in this cell-turnover or regeneration process and good desquamation leads to smoother texture and more radiant skin. Well-ageing effects Fine lines and wrinkles are more noticeable if your skin lacks moisture and feels dry. Moreover, skin regeneration process declines as we age. Since high humidity positively contributes to hydration, lines and wrinkles are less pronounced and the regeneration process supported, therewith leading to more youthful looking skin. Increased sebum production If your skin is oily, high humidity (especially heat thus sweat) can increase sebum production. An overproduction of sebum, especially in combination or oily skin types can make your skin oily and greasy. Moreover, dirt and irritating particles may "stick" better to greasy skin. Acne prone skin Excess sebum and oil caused by high humidity (heat and sweat) increases the risk of clogged pores, break-outs and comedones (blackheads, and whiteheads). Heat or sweat rash Heat rash, or sometimes called prickly heat, sweat rash or miliaria, is a harmless but very itchy or prickling skin rash. It causes small red (raised) spots in places where sweat collects, such as the armpits, back, under the breasts, chest, groin, elbow creases and back of the knees, and the waist. Although uncomfortable, it is usually harmless and improves on its own after a few days. Tips to take care of your skin in humid conditions
LOW HUMIDITY Low levels of humidity most commonly negatively affect your skin. Decrease skin regeneration When your skin is not well hydrated, the skin's regeneration process including shedding of dead skin cells is impaired. This can lead to a more dull, rough or even flaky skin. Dehydrated skin Your skin can get dehydrated or deprived of moisture when exposed to low humidity levels and the skin looks less radiant or glowing. Sings of ageing When your skin lacks moisture, the skin is less plump and wrinkles, fine lines become more visible. Worsening of skin conditions If you have eczema or another problematic impaired barrier related skin condition, low humidity can cause flare-ups. It can also cause or worsen dry skin symptoms like redness, scaliness, rough texture, cracks, itchiness, stinging, burning and the skin might be more prone to irritation and infection. Tips to care care of your skin in low humidity
Take care References

We all know the fairy tail sleeping beauty, however there is actual scientific evidence supporting that good sleep does make you more attractive. Chronic poor sleep quality is associated with increased signs of intrinsic ageing, diminished skin barrier function, lower satisfaction with appearance (1). A variety of studies in the literature have shown that sleep plays a role in restoring immune system function and that changes in the immune response may affect collagen production. (2) Before going into the science supporting that sleep makes you more beautiful, some tips for better sleep and less wrinkles:
A study was conducted with 60 healthy caucasian women, who were categorised as poor quality sleepers [Pittsburg Sleep Quality Index (PSQI) > 5, sleep duration ≤ 5 h] or good quality sleepers (PSQI ≤ 5, sleep duration 7-9 h. Good sleepers had significantly lower intrinsic skin ageing scores by SCINEXA(TM) . At baseline, poor sleepers had significantly higher levels of TEWL (trans-epidermal water loss). At 72 h after tape stripping, good sleepers had 30% greater barrier recovery compared with poor sleepers. At 24 h after exposure to ultraviolet light, good sleepers had significantly better recovery from erythema (redness). Good sleepers also reported a significantly better perception of their appearance and physical attractiveness compared with poor sleepers. (1) Sleep loss could also impact sexual behaviour and collaboration, because sleep is connected with attractiveness, which has an important impact in many social contexts (2). The notion of beauty sleep is actually very important, because sleep deprived people are perceived as more fatigued, less attractive, and even less healthy than when they are rested (3). Sleep deprivation is associated with increased signs of intrinsic skin ageing (fine lines, uneven pigmentation, reduced elasticity) (4), with much slower recovery rates after skin barrier disruption and lower satisfaction with appearance. If we compare people with a normal night sleep with the same persons but sleep deprived, the sleep deprived ones look more fatigued, with hanging eyelids, redder eyes, more swollen eyes, darker circles under the eyes, paler skin, more wrinkles and fine lines around the eyes, the corners of the mouth as being more droopy and more sad (5). In conclusion, the sleep is not connected only with good health (6,7), but is also a link between attractiveness and health. (2,8) Take care and sleep well

It was always believed that the moment we are born, is the moment we are exposed to environmental influences. The truth is that there is ample evidence that already during pregnancy the mothers behaviour: smoking or food has a significant impact on how well we age. We know that all skin needs to be protected against UV and HEVIS by using sunscreen, especially in sun exposed areas from birth onwards.
Although you can not start too early taking care of your skin, the right age to start with a well-ageing skin care routine is actually just post-adolescence for 3 reasons. 1. During adolescence most start with their first cleansing and care routines to remove access of sebum, debris and reduce plus prevent break-outs or comedones. Boys may already shave facial hair. So teenagers or young adults are used to a morning- and evening skin care routine which benefits the overall sense of well-being. 2. Most commonly growth stops when puberty ends and this is the moment the degenerative biological process starts, even though there are no visible signs yet. 3. Prevention of pre-mature ageing skin is the most effective and efficient strategy. SKIN NEEDS CARE There is a movement stating that normal unproblematic skin doesn't need care. I strongly disagree. The choice of products at this age depends of course on the skin type, skin condition, skin health, and environment (like weather conditions, pollution), however the morning care should always focus on protecting every skin type, using suncreen (UV + HEVIS protection) and ideally complimented by anti-oxidants to reduce damaging free radical activity, while the evening routine should at least include proper cleansing (to remove dirt and pollutants), which may be followed by product catering to specific needs, like for example sebum regulating, barrier repairing or hydrating ingredients. I would not make a differentiation between darker or lighter skin in terms of photoprotection, as dark skin only has a natural SPF of 13.3 and light skin of 3.4, hence both not enough to prevent sun damage. However, dark skin has a lower amount of ceramides in the statum corneum and is therefore more prone to trans-epidermal water loss. LAZY SKIN? If you are afraid of spoiling your skin and making it "lazy" using skin care for a long time, know that all effects from a dermo-cosmetic product are 100% reversible, thus temporary. This is regulated by law and to enjoy the benefits from skin care, you need to keep using the products. When you stop, your skin will bounce back to it's original state at least after a full regeneration cycle of about 28 days. A few things to avoid are: sun-damage, especially burns, over-exfoliation (damaged skin barrier) and slugging of oily or acne-prone skin (breakouts). Take care. 
Polynucleotides (PNs) are a type of biomolecule that have recently gained traction in the field of skin care and aesthetic treatments. PNs are composed of multiple nucleotides, which are the building blocks of DNA and RNA. These biomolecules have shown promise in improving the appearance and health of the skin through their ability to stimulate cell growth (activate growth factors), tissue regeneration incl. collagen production, wound healing, fibroblast proliferation and have anti-inflammatory properties.
Polynucleotides (PN) are linear polymers composed of many nucleotide units and they play a key role in the storage and transmission of genetic information. There are two types of polynucleotides (aka nucleic acid) found in nature: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). As mentioned, PN are composed of nucleotides, which consist of 3 parts: a nitrogenous base, a phosphate group. and a five-carbon sugar (2'-deoxyribose in DNA or ribose in RNA). The five base nucleotides are adenine, guanine, cytosine, thymine, and uracil. A DNA molecule consists of two long polynucleotide chains composed of four types of nucleotide subunits: adenine, thymine, guanine, and cytosine, while RNA uses adenine, guanine, cytosine and uracil. REGENERATIVE AESTHETICS Regenerative aesthetics is an emerging branch of regenerative medicine with therapies or products aimed at recapturing youthful structure and function using the body's own systems. Examples of such treatments are platelet-rich plasma (PRP), the use of exosomes or polynucleotides. Dr. Kate Goldie explained soft tissue regeneration fundamentals as following: 1. Regeneration of tissue architecture (structure): tissue composition - component abundance, ratio's, position, density and biomechanics/integrity 2. Regeneration of tissue function: signaling, cell function, cellular components (incl. senescence), gene expression and molecular interaction. The 3 treatment pillars of regenerative aesthetics are: cells, biocues and bio-stimulatory scaffolds. Key superficial soft quiescent cells are the fibroblasts and adipose derived stem cells. One of the big advantages of regenerative aesthetics is by using the body's own system, the results are natural and focused on "skin health" (function) and "skin quality" (appearance). POLYNUCLEOTIDES IN REGENERATIVE AESTHETICS Polynucleotides are most often natural, highly purified DNA molecules extracted for example from trout gonads and activate specialised cells called myofibroblasts and adipocytes. PN containing devices act as short time temporary fillers thanks to the viscoelasticity of the long DNA fragments and improve skin well‐being (cell growth) and steady self‐repair (tissue regeneration). Studies support their dermal reactivating properties or their efficacy as “bioreactivating primers” of skin. The final outcome is more natural and in‐depth tissue regeneration and a healthier look of the skin: a more radiant complexion, even skin tone, reduced appearance of fine lines, wrinkles and sagging, faster wound healing, improved pore size and skin thickness, elasticity and hydration. Furthermore, PNs are generally well-tolerated by the skin and have a low risk of adverse effects. Their effectiveness may vary depending on the individual's skin type, age, and overall health. The long-term effects of PNs on the skin are not yet fully understood, and more research is needed to determine their safety and efficacy. There are various brands available which use polynucleotides in their (meso-) injection gels. For example Mastelli Srl, Italy NEWEST® (Polynucleotide and Hyaluronic Acid) for bio-revitalization, BR Pharm HP Cell Vitaran Skin Healers, NUCLEADYN® or Nucleofill®. One brand of (synthetic) polynucleotide-based skin care products is Yuva by Dr. Devgan Scientific Beauty. The Yuva line includes a range of products formulated with PNs, such as the Yuva Serum and the Yuva Enhancer. These products are marketed as being able to provide hydrating, anti-aging, and skin-rejuvenating benefits. THE FUTURE OF POLYNUCLEOTIDES While polynucleotides have many benefits, they also have some drawbacks. One of the primary limitations is their instability in certain environments. This instability can make it difficult to synthesize and manipulate polynucleotides in the lab. Moreover their are limitations, risks and ethical concerns harvesting or using (human identical) PN's, and long-term safety and efficacy data is not conclusive. However, PNs are a promising area of research in the field of skin care and aesthetic treatments and regeneration. We can expect to see further advancements in the development of PNs-based products and treatments. PNs are already used in combination with other biomolecules, such as hyaluronic acid, growth factors and anti-oxidants and used in combination with other treatments. For a personal recommendation on which aesthetic treatment is most suitable to aesthetically regenerate your skin, please consult an experienced board certified dermatologist, plastic surgeon or cosmetic doctor. Take care. 
Glycation is one of the basic root causes of endogeneous (intrinsic) skin ageing and a very challenging one or almost impossible one to reverse. Glycation is an ageing reaction which begins in early life, developing clinical symptoms at around 30, and progressively accumulates in tissues and skin due to the glycated collagens that are difficult to be decomposed. Glycation occurs naturally in the body when sugars react with proteins and lipids to form advanced glycation end products (AGEs). AGEs can be exogenously ingested (through food consumption), inhaled via tobacco or endogenously produced and formed both intracellularly and extracellularly. AGE modifications lead to dermal stiffening, diminished contractile capacity of dermal fibroblasts, lack of elasticity in the connective tissues, contribute to hyperpigmentation and a yellowish skin appearance. The formation of AGEs is amplified through exogenous factors, e.g., ultraviolet radiation.
AGEs cause changes in the skin through 3 processes:
One study published in the Journal of Investigative Dermatology found that levels of AGEs were higher in the skin of older individuals compared to younger ones. The study also showed that there was a correlation between the level of AGEs and the severity of skin ageing. This suggests that inhibiting the production or accumulation of AGEs in the skin is a potential target for anti-ageing interventions or skin ageing management. AGEs are complex and heterogeneous, more than a dozen AGEs have been detected (however not all) in tissues and can be divided into three categories according to their biochemical properties. AGEs are formed through four pathways:
GLYCATION INHIBITION IS KEY AGEs can be crosslinked through side chains to form a substance of very high molecular weight, which is not easily degraded. The consequences from skin glycation are irreversible. This makes prevention or inhibition of the process the best potential strategy to maintain skin health and ageing skin management. One way to do this is by altering the diet to reduce the intake of sugars and carbohydrates, which are known to contribute to glycation. Several studies have found that reducing sugar intake can result in significant improvements in skin health, including reducing wrinkles and improving skin texture. 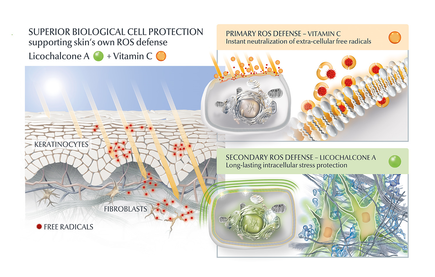
AGE inhibitors
Another potential strategy is the use of topical agents that inhibit the formation or accumulation of AGEs in the skin. One study published in the Journal of Cosmetic Science found that a cream containing carnosine, a peptide that inhibits glycation, improved skin elasticity and reduced the appearance of wrinkles in individuals with ageing skin. Skincare containing NAHP or Acetyl Hydroxyproline inhibits the formation of AGEs significantly (in vitro), most likely through a mechanism where NAHP competes with the proteins for the sugar. Finally, NAHP sacrifices itself in place of the proteins and gets (at least partially) glycated. NAHP also prevents loss of cellular contractile forces in a glycated in vitro dermis model and counteracts the diminished cell-matrix interaction that is caused by glyoxal-induced AGE formation. Anti-Oxidants Moreover, I would suggest to combine those ingredients with an ingredient like Licochalcone A. Numerous high ranked publications support that Licochalcone A protects cells from oxidative stress mediated by e.g. UV and HEVIS (blue light) induced reactive oxidative species (ROS). Due to the activation and nuclear translocation of the transcription factor NrF2, the expression of anti-inflammatory, antioxidant and detoxifying enzymes are induced. These enzymes protect the skin cells (like keratinocytes and fibroblasts) from ROS-induced damage, like lipid peroxidation and DNA as well as protein damage. If Licochalcone A is combined with L-Ascorbic Acid, (the most active form of Vitamin C), it supporting skin's own collagen production, provides superior biological cell protection amongst other relevant benefits. My absolute favourite product is Eucerin Hyaluron-Filler Vitamin C Booster which I use daily as a serum in my morning routine. GLYCATION AND SKIN HEALTH Acne In addition to its role in ageing, glycation in the skin has also been linked to a range of skin health problems. One study published in the Journal of Cosmetic Dermatology found that the level of AGEs in the skin was significantly higher in individuals with acne than in those without acne. The study also showed that treating acne with a topical antibiotic significantly reduced the levels of AGEs in the skin. Atopic Dermatitis Another study published in the Journal of Investigative Dermatology found that individuals with atopic dermatitis had higher levels of AGEs in their skin than healthy individuals. This suggests that glycation may play a role in the development of inflammatory skin conditions. Diabetes + Woundhealing The correlation between high sugar levels and skin ageing can be seen in diabetic patients, where one-third of this population has skin complications. A prominent feature of ageing human skin is the fragmentation of collagen fibers, which severely damages the structural integrity and mechanical properties of the skin. Elevated levels of MMP-1 and MMP-2 and higher crosslinked collagen in the dermis of diabetic skin lead to the accumulation of fragmented and crosslinked collagen, thereby impairing the structural integrity and mechanical properties of dermal collagen in diabetes. Collagen crosslinking makes it impossible for them to easily repair, resulting in reduced skin elasticity and wrinkles. Keratinocytes and fibroblasts are the main cells involved in wound healing, but due to the high glucose (HG) microenvironment in diabetics, the functional state of these cells is impaired, thereby accelerating cellular senescence (programmed cell death). Conclusion We can't completely stop the glycation process, therefore it's important that we inhibit it from a young age onwards, hence monitor the sugar intake of our children, use daily SPF and invest in good dermo-cosmetic products containing ingredients like NAHP and powerful anti-oxidants like L-Ascorbid Acid (Vitamin C is needed for the production of collagen) and Licochalcone A (also anti-inflammatory). Preventing signs of ageing, specifically caused by glycation is most effective. If your skin shows (advanced) signs of ageing, you can get visible improvement using skin component (hyaluron, collagen and elastin) bio-stimulating ingredients like Retinol, Bakuchiol, Arctiin, Creatine or Glycine Saponin. Consult your dermatologist if you wish to improve your skin's appearance or skin health issues. Take care Special thanks: Ph.D. dr Julia M. Weise Manager Biological Testing & Dorothea Schweiger Lab Manager Facial Skin Biology Beiersdorf HQ Hamburg 
We all learned that sleeping in make-up is the ultimate skincare sin. What is bad about it is when you go 24 hours without washing your face and end up going to bed leaving your day-time make-up on. Over the course of the day, our skin accumulates pollutants, dirt and dead skin-cells.
If dirt and pollutants are left on the skin, they may cause micro-inflammation and contribute to premature ageing skin via a process called inflamm-aging and free-radical damage which is a major contributor to skin-ageing. The combination of both micro-inflammation and free-radical damage is called ox-inflammation. We should aim to reduce or preferably avoid it. Pollution, dirt and sebum (oils) can impact the skin's healthy pH balance and thus lead to a weakening of the skin barrier function, more sensitive skin, dehydration, slowed down skin-cell renewal process and thus ageing. Not removing dead skin cells together with dirt increases the risk of clogged pores. Make-up itself usually doesn’t contain harming ingredients. Coloured micro-pigments actually provide additional sun-protection. Make-up or foundation itself is thus not the problem, however the fact that we don’t cleanse our skin after a busy day and/or evening is what could make us age faster. Not doing your PM cleanse and care routine is anyway a missed opportunity to support your skin’s night-time recovery with beneficial active ingredients. If you go out in the evening, take the opportunity to cleanse before getting ready and get rid of debris which was accumulated during day-time. Don’t worry about falling asleep in your make-up once or twice. Just don’t make it a habit. I would always aim to remove eye make-up. Sleeping in full eye make-up (mascara, liner, eyeshadow) increases the risk of an eye-inflammation, redness and corneal abrasions. Waking up with “panda-eyes” filled with black rheum or goop isn’t pretty either. Take care 
Vitamin C is a "must have" skin care ingredient our skin needs at any age.
One of the best researched skin care ingredients and proven to be very beneficial for skin is Vitamin C. Our skin uses Vitamin C as an anti-oxidant and the dermal fibroblasts need Vitamin C for the production of collagen. Two very good reasons to add this ingredients into your daily skincare routine whether you are twenty or eighty. Moreover, our skin depends on us for the needed supply, as our skin is not able to produce Vitamin C itself. We can either include enough Vitamin C in our diet or apply Vitamin C topically there where we need it the most. Usually this is the skin which is exposed to (sunlight) as this increases damaging free radical activity in our skin. An active form of vitamin C can reduce the free radical activity, which we call anti-oxidative effect. There are 4 things to consider when buying a skincare product containing Vitamin C:
Day or night? Some recommend to use Vitamin C during the night, as the active form of Vitamin C will oxidize in daylight. Hence, your skin can benefit from the Vitamin C longer during the night. I would recommend Vitamin C to be used during daytime (thus added to your morning routine), as we need protection from damaging free radicals the most during daytime and the oxidization of Vitamin C is actually a sign that the ingredient is doing it’s job! It’s even better to add Vitamin C both to your day & night time skincare routine. Is L-Ascorbid Acid enough? Vitamin C is counteracting free radicals from UV light. However, UV is not the only damaging light form as there is also High Energy Visible Light or abbreviated HEVIS. This penetrates even deeper into the skin where also the dermal fibroblasts reside. The dermal fibroblasts are our collagen and hyaluronic acid producing cells and a key target in an effective anti-ageing skincare strategy. Lichochalcone A (Licorice-root extract) has proven to be the most potent anti-oxidant to protect the dermal fibroblasts and neutralize free radicals from HEVIS. Moreover, Lichocalcone A increases Glutathione, which is a skin’s own anti-oxidant. Licorice-root extract is an anti-ageing hero. Summary The combination of Vitamin C and Lichocalcone A will protect our skin and dermal fibroblasts from free radical damage by UV and HEVIS and will provide superior biological cell protection in comparison to Vitamin C only. For me this is a good reason to use a product containing both ingredients as a first step after my cleansing routine in the morning. If you have sensitive eyes, I recommend to use an eye care prior, which will form a barrier to help to prevent the low pH Vitamin C product to migrate into the eye area. Afterwards you can use the other products of your skincare routine. I would like to put emphasis on using a SPF of 30 or higher during the day. This will not only help to protect your skin, but also support the anti-oxidative benefits and make them last longer. Hope this was helpful. Take care! 
One of the frequently asked questions is, if it's necessary or if there is a benefit using a special eye care or cleansing products. Yes, there is!
As I mention in many of my previous posts, the right pH-level is very important for healthy skin. Skin usually prefers a pH of around 5. However there are some area's where the skin's natural pH balance is a little bit different. One of those area's is the area around the eyes. The preferred pH-level there is around 7, thus less acidic and more alkalic in comparison to your regular cleansing or care product for face or body. This is one of the most important reasons why I would recommend to use a special eye make-up remover and eye care product, as they are adjusted to the pH level most suitable for use in the eye area. Furthermore, special eye products are tested and proven to be safe when used around the eyes, while it isn't always recommended or proven for a regular face product. Some care products have a tendency to "travel" or migrate into the eye area. Even when not directly applied around the eyes, they might end up there. A special eye care product can form a "barrier" and thus help to prevent that unwanted products move to the eye area and cause irritation. I would particularly recommend the use of an eye cream when using other products containing gold standard anti-ageing active ingredients like Vitamin A, C (or derivatives of both), Hydroxy Acids (Alpha, Beta or Poly), when you have experienced some sensitivity of the eyes or eye area in the past or have a more problematic skin type. Eye care products preferably should not contain Vitamin C (L-Asorbic Acid or related) as it requires a low pH value of <4 to be active and do it's job properly. Eye care products with Vitamin C therewith are either too acidic to be used in the eye area or alternatively too alkalic for the Vitamin C to be effective. Safe to use in the eye area are products containing Hyaluronic Acid. Although "Acid" is in the name, Hyaluronic Acid isn't acidic. One of it's key functions is attract and bind water, which usually has a pH of ~7. Take care! 7/22/2018 Comments Chemical or mechanical exfoliation
We can support's skin natural exfoliation process in various ways, for example with mechanical or chemical exfoliation.
Desquamation (shedding of skin cells thus exfoliation) is an important part of the skin's natural regeneration or renewal process. Already in our twenties, this process slightly, however increasingly starts to slow down (Kligman 1983). As a result, the cells on the surface of our skin (corneocytes) become bigger (Kligman 1989) and a little disorganised. This leads to a duller appearance (loss of radiance) and a more rough texture of our skin. A very comprehensive comparison of both methods:
The word "acid" unfortunately sounds very harsh and skin-unfriendly. Many acids are actually skin's own, like for example lactic acid is a skin's own natural moisturising factor (NMF) and so is hyaluronic acid. The level of NMF's decrease as we age and our skin my lose the ability to maintain well hydrated. Many years ago the benefits of lactic acid were capitalised by using baths filled with donkey milk. Citric acid is commonly used in skin care products and toners to balance skin's pH. Gluconolactone is only gradually penetrates skin and is very gentle.
It's unfortunate that "acids" have such a negative connotation, as our skin (healthy and problematic) can benefit if we use them regularly. Moreover, I prefer this method over mechanical exfoliation for all skin types, however particularly if you have dry skin, acne- or redness prone skin, sensitive skin or mature skin. The risk of exfoliation is over-exfoliation. Over-exfoliation is damage of our skin barrier and the symptoms are very comparable to dry or (hyper) sensitive skin symptoms, which are: redness, irritation, tightness, excessive dryness, dry patches, flaking skin, uncomfortable stinging, or even burning sensation. Whenever you experience one or more symptoms of over-exfoliation, it's recommended to reduce the number of times you exfoliate and support the skin barrier repair with a moisturiser. Hope you enjoy healthy skin & take care. 7/12/2018 Comments Relation skin pH and ageing process
A high pH value contributes to premature ageing skin!
A study published in British Journal of Dermatology showed that women with an alkaline stratum corneum (outer layer of the skin) developed more fine lines and crow's-feet (wrinkles at the outside corner of the eyes) than those with acidic skin over an eight-year period. This might be in part because an alkaline epidermis (top layer of the skin) tends to be drier and more fragile than an acidic one. Irritants can enter the skin and water can evaporate more easily. People with hydrated skin showed a 50% lower rate of wrinkling than those with dry skin. If the acid mantle is not intact, it can make skin more susceptible to inflammation (inflammaging) and lowered enzymatic activity, which again increases the risk of development of signs of ageing. Last but not least, alkaline skin is more prone to sun damage thus photo-aging, because its protective barrier has been weakened. pH balance is fragile. I just mentioned that alkaline skin tends to be drier, however it’s also known that the oils secreted by our skin impact skin’s pH by increasing it. This is one of the reasons that oily skin types can be more prone to acne, as the skin’s pH influences it's microflora. That's a topic for another blog post. You’ve maybe seen some of my previous posts on skin’s pH and it’s actually one of my major topics. This is because healthy skin starts with an optimal pH balance. Click below in the featured categories on “Skin pH” if you like to learn more about pH. If there is a specific topic you are interested in missing, please place a comment below and I will see to it that I address it. Take care. 7/10/2018 Comments Facial toners redundant?
Recently I've read an article in which facial toners were called a redundant step in the cleansing routine. They would not serve any purpose anymore and would be “old-fashioned". I disagree, and will explain why.
Particularly when you prefer wet facial cleansing (water has a pH of 7-8), your skin’s pH goes up and you may consider using a toner to bring it back to normal (~5) before using a moisturizer or serum. This also applies if you use an alkaline cleanser or micellar water. It is common that products which are suitable to be used around the eyes, like micellar water, are adapted to a more “eye-friendly” and less “skin friendly” pH of ~7. Skin prefers a pH of ~5. In my humble opinion, toners are a very important step in every a.m. and p.m. skin care regimen for both healthy and particularly problematic skin types. They refresh, remove left-over debris and make-up and moreover instantly rebalance skin’s pH value. A balanced pH value is the cornerstone for healthy skin. An optimal pH supports skin's microbiome (microflora or "ecosystem") and barrier function. Furthermore, the use of a toner usually helps the penetration and thus efficacy of your care product! Alternatively, you can use “chemical” exfoliating lotions or pads which contain AHA (glycol, citric and lactic acid), BHA (salicylic acid), PHA (gluconotactone), etcetera. Just be careful using them around the eyes or even avoid this area. Hope you enjoy healthy skin & take care. 7/8/2018 Comments Wellness not skin friendly?
Long showers and bathing, though considered wellness or “home spa” experience, may actually cause a major disruption to our skin’s pH and skin barrier. The same goes for swimming (in salty sea or pool with chlorine).
These activities increase the risk of skin sensitivity and dryness. Water has a pH of 7-8 while our skin’s optimal pH balance requires a pH ~5. To make long showers and bathing a more a skin friendly experience, it is highly recommended to use non-alkaline or slightly acidic gentle cleansers (preferably with a pH ~5). If you have time, apply afterwards a pH rebalancing body lotion or butter. Your skin will surely appreciate it. I would personally never even consider applying an alkaline body product, as it might take the skin several hours to rebalance it’s pH on a large surface. Instead of hydrating your skin, you may in fact increase the risk of water loss; which is a waste of precious time and (expensive) product. Surely, I don't want to discourage anybody from enjoying swimming, relaxing long showers and baths! Just give your skin a "little love" afterwards. 
Although I am writing this blog post as a private person, the skin care company I am working for as a senior global medical manager was one of the first, if not the first to understand the importance of optimal pH balance of the skin, the cornerstone for healthy skin of face and body. Every day, we expose our skin to pH disruptors, like water (usually a pH of 7-8), alkaline soap, sun, pollution and many more. Even though our skin is resilient and eventually will go back to it’s optimal pH (»5), it is common sense and beneficial for our skin’s health to support our skin with a suitable skin care regimen, reducing disruption and promoting pH-balance.
WHAT IS pH? pH literally means the power (or concentration) of hydrogen atoms in a substance. Substances with a higher concentration of Hydrogen atoms than Hydroxide molecules have a greater acidic 'power' (acids), while those with lower H+ (Hydrogen) and higher OH- (Hydroxide) concentrations have less acidic power (bases). The pH scale runs from 0 to 14: 7 is a neutral pH, values below 7 (6-0) are increasingly acidic while values above 7 (8-14) are increasingly basic. There is a 10-fold difference in concentration between these values, meaning that pH 0 is 10 times stronger than pH 1, 100 times stronger than pH 2, and 10,000,000 times stronger than pH 7. With other words; a slight increase in pH, can make a big difference for your skin. SOME PHYSIOLOGICAL FUNCTIONS OF SKIN pH
SKIN BARRIER pH plays a fundamental role in the skin's barrier, acid mantle or buffer, which is formed by secretions from sweat and sebaceous glands as well as the breakdown of fatty acids by beneficial micro-flora. The barrier functions like an invisible protective veil (hydrolipid film) that protects skin’s lipids, moisture loss and unwanted allergens, pollution and bacteria. The acid mantle is at its strongest and most optimal balanced when the skin is slightly acidic, with an average pH of about 5.5 (ranging anywhere from 4.5 to 6.2). However, different areas of the body have slightly different requirements and different optimal pH ranges. The skin of women is slightly more acidic than skin of men. pH BIORYTHM Skin surface pH decreases during normal working hours with maximum values in the afternoon and minimum values during night time. You’ve probably heard that our skin regeneration is most optimal during night time? Indeed, our skin’s pH plays a key role in this too. In separate posts I will address various specific skin pH related topics, as I could go on forever about pH. There is so much to share on this topic (and still to discover) and balanced pH is essential for healthy skin, from cradle to grave, for all skin types & all phototypes. Please check out my other posts, if you are interested. Hope you enjoy healthy skin & take care. 7/7/2018 Comments Skin's pH influences hydration
Our skin’s pH plays an important role in many processes influencing skin hydration.
Increased pH (more alkaline >6) values correspond with an increase in trans-epidermal water loss, which we refer to in studies as TEWL, one of the most significant indicators of a good epidermal barrier function. The epidermal barrier protects our skin against (excessive) loss of water and thus protects the maintenance of correct hydration. The skin pH promotes the correct organization of the matrix lipids too, by regulating their surface structure and stability. All leading up to better hydrated skin. It makes sense to pay attention to pH when buying your cleansing and care products. Hope you enjoy healthy skin & take care. 2/27/2018 Comments Malodorous armpits
Ever wondered why armpits are smelly, even when using a luxurious and fabulous fragranced deodorant?
The answer might be in your skin’s pH value. Sweat secreted by the glands under our armpits increases local skin’s pH value. This negatively influences it’s microflora, also called skin’s microbiome. A high and unhealthy pH value encourages certain odor-producing bacteria to flourish. Hence, a more alkaline (high) pH value due to (excessive) sweat, does cause smelly armpits or other malodour in other body areas. No fragrance can mask or does counteract this process effectively. That's why we can still suffer from smells armpits while using deodorant. What you can do to balance pH and keep armpits dry Opt for a skin pH friendly deodorant (~5) which reduces perspiration. With less sweat and by maintaining skin’s optimal pH balance, you will automatically reduce nasty bacterial activity. Keep armpits clean Regularly wash away the alkalising sweat and bad bacteria with their smelly by-products. Some skin experts recommend using antibacterial soap. It will reduce all bacterial activity, good and bad. I would rather target only the unwanted bacterial activity with a more skin-flora friendly approach by using gentle cleansing products with a pH value ~ 5. This keeps good bacteria on the skin (we need them for balance) and discourages growth of overly active odor producing bacteria. Make sure that your armpits dry down properly before and after applying a thin layer of deodorant. Shaving Reserve shaving armpits or the use of other hair removal devices for night-time. Gently cleanse and dry your skin prior. Removing hair in the evening and postpone using a deodorant till the next morning will allow your skin to recover a few hours. Mild abrasive hair removal methods can cause micro-injuries to the skin barrier which makes our skin more prone to irritation. Anyway, most of us are less bothered by odor, or at least less aware while sleeping, and we depend more on our pH friendly deodorant to do a good job during day time. Use all skin care products with modesty, except sunscreen! Hope you will enjoy a great summer without malodorous armpits. |
CategoriesAll Acne Ageing Aquatic Wrinkles Armpits Biostimulators Blue Light & HEVIS Cleansing CoQ10 Cosmetic Intolerance Syndrome Deodorant Dermaplaning Diabetes Dry Skin Evidence Based Skin Care Exfoliation Exosomes Eyes Face Or Feet? Facial Oils Fibroblast Fingertip Units Gendered Ageism Glycation Gua Sha Hair Removal Healthy Skin Heat Shock Proteins Hormesis Humidity Hyaluron Hyaluronidase Hypo-allergenic Indulging Jade Roller Licochalcone A Luxury Skin Care Lymphatic Vessel Ageing Malar Oedema Menopause Mitochondrial Dysfunction Mood Boosting Skin Care Neurocosmetics Ox Inflammageing PH Balance Skin Photo Biomodulation Polynucleotides Psoriasis Regenerative Treatments Review Safety Scarring Sensitive Skin Skin Care Regimen Skin Flooding Skin Hydration Skin Senescence Skip-Care Sleep Slugging Sunscreen Tanning Under Eye Bags Vitamin C Well Ageing Skin Care Wound Healing Wrinkles
Archives
April 2024
|


 RSS Feed
RSS Feed
