
Blue light, is also known as high-energy visible (HEV) light and is the most energetic part of the visible light spectrum (380 - 700 nm) with wavelengths ranging from indigo or ultramarine light 420-440 nanometers, blue light 450-495 nanometers to cyan light 480 - 520 nanometers. Blue light has lower energy than ultraviolet (UV) radiation (280–400 nm) and can reach further into the dermis, up to the depth of 1 mm. [1] Sunlight is the primary natural source of blue light. Up to 50% of the damaging oxidative stress in human skin is generated in the VIS spectrum and the other 50% by UV light [2], contributing to premature ageing, ox-inflammageing and hyperpigmentation like age spots.
Blue light from electronic devices The use of electronic devices has led to increased exposure to artificial blue light sources, however the amount of blue light emitted during the conventional use of electronic devices is by far not enough to trigger harmful skin effects. If you sit in front of a monitor uninterrupted for a week at a distance from the screen of approximately 30 cm, this would be the same as the blue light intensity of spending one minute outside on a sunny day in Hamburg Germany at around midday at midsummer. If you hold a smartphone right next to the skin, the intensity does increase, but it would still take approximately 10 hours of uninterrupted use to match the effect on the skin of just one minute of sunlight. The emissions from electronic devices are barely noticeable in comparison to natural blue light directly from the sun and are, thus negligible. However, blue light or HEV light from sunlight can be harmful for skin. Dr Ludger Kolbe Chief Scientist for Photobiology and his team at Beiersdorf AG did pioneering research regarding the harmful effects of HEVIS. [3-4] I would also like to take the opportunity to debunk an important myth at the start of this article as infrared or near infrared light does not induce damaging free radicals (even in high amounts), there is no such thing "infra-ageing" as a result or IR and in fact red light photobiomodulation supports skin rejuvenation. Read more Direct effects of blue light and HEV Light on skin Blue light and HEV light can have both beneficial and detrimental effects on the skin. The most significant direct effects are mediated through their interaction with chromophores, such as flavins, porphyrins, and opsins, which can trigger the overproduction of reactive oxygen species (ROS), reactive nitrogen species (RNS). and hyperpigmentation. Reactive oxygen and nitrogen species cause DNA damage and modulate the immune response. [1] This oxidative stress can lead to: Photo-ageing: Exposure to blue light and HEV light can induce premature skin aging, causing wrinkles, fine lines, and loss of elasticity. Hyperpigmentation: Blue light and HEV light can stimulate melanin production, leading to uneven skin tone and the development of age spots or other forms of hyperpigmentation. DNA Damage: The ROS and RNS generated by blue light and HEV light can cause DNA damage, plus potentially increase the risk of skin cancer. Inflammation: The oxidative stress triggered by blue light and HEV light can cause an inflammatory response in the skin, exacerbating conditions like acne, eczema, and psoriasis. Molecular and physiological mechanisms of direct blue light effects on the skin [1]
Indirect effects of blue light and HEV Light on skin Blue light and HEV light can also have indirect effects on the skin by disrupting the body's circadian rhythms. This occurs via both the central mechanism, which involves stimulation of light-sensing receptors located in the retina, and via the peripheral mechanism, which involves direct interaction with skin cells. By disrupting the normal circadian rhythm, blue light can negatively affect the skin's natural overnight repair and regeneration processes. [1] The circadian rhythm has been shown to affect multiple cellular and physiological processes occurring in the skin:
Molecular mechanisms of indirect effects of blue light on the skin [1]
Ideal daytime & nighttime skin care regimen When considering cosmetic interventions, a strategy of daytime protection plus defense and night-time repair may be optimal. The skin's own repair mechanisms, such as base excision repair and nucleotide excision repair, attempt to mitigate blue light induced DNA damage. [12] Daytime protection plus defense Of course prevention and/or reduction of blue light exposure from sunlight is key. Reduce the time spent on electronic devices, especially before bedtime, can help minimize the disruption of circadian rhythms and the indirect effects of blue light and HEV light on the skin. Against premature ageing and hyperpigmentation an evidence based effective approach could be the daily use of tinted broadband sunscreen preferably containing Licochalcone A (the most effective anti-oxidant reducing damaging free radical activity from both UV and blue light and moreover protects against collagenase MMP-1 expression) strengthening skin's biological defense [4-5-6-7], while iron oxides in colour pigments provide physical protection against blue light (like zinc oxide and titanium dioxide). Against hyperpigmentation there are (tinted) sunscreens which on top contain the most potent human tyrosinase inhibitor found in dermatological skin care called Thiamidol® [8-9] and one of the 3 ingredients in the "new Kligman Trio" (NT) [18] and Glycyrrhetinic Acid which supports skin's DNA repair and skin pigmentation [10] and inhibits hyaluronidase activity (HYAL1). Most regular sun filters used in sunscreen don't offer any protection against blue light, however according to the website of BASF the chemical UV filters Tinosorb® A2B and Tinosorb® M can reduce the exposure to blue light. [11] Scattering and absorption of blue light [5] The penetration depth of visible light is influenced by the reflection, scattering, and absorption mediated not only by the skin’s physical barrier but also by the VL chromophores in the skin and Fitzpatrick skin or photo-type (FST). The primary VL-scatter and absorption molecules in the skin include hemoglobin, melanin, bilirubin, carotene, lipids, and other structures, including cell nuclei and filamentous proteins like keratin and collagen. Melanin and keratins are the primary VL absorbers and scatterers in the epidermis, while hemoglobin is the dominant absorber, and collagen is the major VL scatter in the dermis. Melanin's absorption spectrum ranges from 200 to 900 nm, with the peak absorption varying based on melanin moiety.. This means that individuals with darker skin types, which have higher melanin content, are more prone to hyperpigmentation from blue light or VIS due to the greater absorption and scattering of VIS in their skin on top of the previously mentioned higher levels of tyrosinase–DCT complexes leading to increased melanogenesis, leading to both transient and long-lasting pigmentation [13], dependent upon the total dose and exacerbation of melasma especially in individuals with FSTs III to VI. Blue light tanning Recent data demonstrate synergistic effects between VL and UV-A on erythema and pigmentation. VL-induced pigmentation is more potent and more sustained than UVA1-induced pigmentation in darker skin tones.Typically, three mechanisms are involved in the responsive reaction of melanocytes to VL, with increased melanin content: immediate pigment darkening (IPD), persistent pigment darkening (PPD), and delayed tanning (DT). [15] Read more. VL can also exacerbate post inflammatory hyperpigmentation (study with FST IV and V). [16] Blue light therapy While the detrimental effects of blue light and HEV light on the skin have been well-documented, these wavelengths have also shown promise in the treatment of certain skin conditions. In controlled clinical settings, blue light has been used to: Treat Acne: Blue light can reduce the growth of Propionibacterium acnes, the bacteria responsible for acne, and has an anti-inflammatory effect. Manage Psoriasis and Atopic Dermatitis: Blue light has been found to have an anti-inflammatory and antiproliferative effect, making it potentially beneficial for the treatment of these chronic inflammatory skin diseases. Reduce Itch: Some studies have suggested that blue light may help alleviate the severity of itching in certain skin conditions. The optimal protocols for blue light therapy are still being developed, and the long-term safety of this treatment modality requires further investigation and should not be initiated without HCP recommendation and monitoring. Vitiligo: Blue light therapy via LEDs can stimulate repigmentation in patients with vitiligo with minimal adverse events, however larger studies are needed. [17] Overall, the research suggests that prolonged or excessive exposure to high-energy blue light, can have negative long-term effects on skin structure, function, and appearance in all phototypes. As our understanding of the individual variations in skin's response to blue light exposure deepens, the development of personalised or tailored effective solutions become increasingly more tangible. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References
Comments

Mitochondria are the "powerhouses" or "lungs" of our cells and bioenergetic semi-autonomous organelles with their own genomes and genetic systems. [1] They are responsible for generating the energy that fuels a wide range of cellular processes in the skin, including cell signaling, pigmentation, wound healing, barrier integrity [2], metabolism and quality control. [3] Mitochondria exist in each cell of the body. Their primary role is cellular respiration; a process converting the energy in nutrients (like glucose) into a usable form of energy called ATP or Adenosine Triphosphate. Mitochondria are particularly abundant in the skin, reflecting the skin's high metabolic demand. When the functionality of mitochondria is impaired or declines, it impacts skin's vitality, health and beauty. Mitochondrial dysfunction is 1 of the 12 hallmarks of skin ageing.
The skin is particularly susceptible to mitochondrial stress due to its constant exposure to environmental insults, such as UV radiation, pollution, and other oxidative stressors. These factors can damage mitochondrial DNA, leading to increased production of reactive oxygen species (ROS) and disrupting the delicate balance of cellular processes. [4] In aged post-mitotic cells, heavily lipofuscin-loaded lysosomes perform poorly, resulting in the enhanced accumulation of defective mitochondria, which in turn produce more reactive oxygen species causing additional damage (the mitochondrial-lysosomal axis theory). [5] Optimal mitochondrial function is indispensable for sustaining the specialized functions of each cell type, like keratinocyte differentiation, fibroblast ECM production, melanocytes melanin production and distribution, immune cell surveillance, sebocytes and adipocytes. [6] Mitochondrial dysfunction is both directly and indirectly linked to chronological ageing and photo-ageing. [7] As mitochondrial function declines, the skin's ability to regenerate and repair itself is decreased. [2=1] This results in visible signs of aging, such as wrinkles, loss of elasticity, dryness, uneven pigmentation, melasma, age spots, lipomas, impaired wound healing. [2-4-5-8-9] Mitochondrial dysfunction also has been implicated in skin conditions like acne, eczema, lupus, psoriasis, vitiligo, atopic dermatitis and even skin cancer. [10] Ageing is associated with changes in mitochondrial morphology, including [6]
Good mitochondrial function or metabolism: [7]
Dysfunctional Mitochondria: [7]
Mitochondrial proteins Mitochondria contain >1,100 different proteins (MitoCoP) that often assemble into complexes and supercomplexes such as respiratory complexes and preprotein translocases. The chaperones Heat Shock Proteins HSP60-HSP10 are the most abundant mitochondrial proteins. [3] Small heat shock proteins form a chaperone system that operates in the mitochondrial intermembrane space. Depletion of small heat shock proteins leads to mitochondrial swelling and reduced respiration. [14] Mitochondrial hyperpigmentation Emerging research has shed light on the intricate relationship between mitochondrial dysfunction and the development of hyperpigmentation, a condition characterized by the overproduction and uneven distribution of melanin in the skin. One of the key mechanisms underlying this connection is the role of mitochondria in the regulation of melanogenesis, the process by which melanin is synthesized. Mitochondria are involved in the production of various cofactors and signaling molecules that are essential for the activity of tyrosinase, the rate-limiting enzyme in melanin synthesis. [15] When mitochondrial function is impaired, it can lead to an imbalance in the production and distribution of these cofactors and signaling molecules, ultimately resulting in the overproduction and uneven deposition of melanin in the skin. [15] This can manifest itself as age spots, melasma, and other forms of hyperpigmentation. The link between mitochondrial dysfunction and hyperpigmentation has been further supported by studies on genetic disorders that involve mitochondrial dysfunction, such as mitochondrial DNA depletion syndrome. In these conditions, patients often exhibit a range of pigmentary skin changes, including patchy hyper- and hypopigmentation, as well as reticular pigmentation. [16] Mitochondrial crosstalk and exosomes Mitochondria can crosstalk and move beyond cell boundaries. [17] Mitochondria-derived material might be transferred to neighboring cells in the form of cell-free mitochondria or included in extracellular vesicles [18-19]. This process supports cellular repair and contributes to vital mitochondrial functions. Besides restoring stressed cells and damaged tissues due to mitochondrial dysfunction, intercellular mitochondrial transfer also occurs under physiological and pathological conditions. [20] The transfer of active mitochondria from mesenchymal stem cells (MSCs) has been identified as a repair mechanism for rejuvenating damaged skin fibroblasts. [21] MITOCHONDRIAL SUPPORT Q10 or Coenzyme Q10 (CoQ10) Q10 is part of the mitochondrial respiration chain and essential for cellular energy production. About 95% of our cellular energy is generated with support of Q10, which is produced by the human body itself. During skin ageing, both the cellular energy production and levels of Q10 are declined. Q10 is a powerful anti-oxidant [22], thus protecting cells from oxidative stress and damage and has proven to be able to "rescue" senescent cells by decreasing elevated senescent markers like p21 levels and β-Galactosidases positive cell numbers (in-vitro). Q10 is bio-active, increasing collagen type I and elastin production. [23=8] Q10 can be supplemented via nutrition, however also via topical application and is considered an evidence based active ingredient in skin care products. Ubiquinol (reduced form) shows higher bioavailability compared to ubiquinone (oxidized form). [23] Glutathione Glutathione is formed in cell's cytoplasm from glutamic acid, cysteine and glycine. It is present in 2 forms: reduced (GSH) and oxidized (GSSG). Reduced GSH is an active anti-oxidant, while the presence of inactive GSSG is increased under oxidative stress. The ratio between GSH and GSSH is considered a measure of oxidative stress. Glutathione participates in redox reactions, acts as co-factor of many anti-oxidant enzymes and is the most important non-enzymatic anti-oxidant, essential for synthesis of proteins and DNA. Low Glutathione results in accelerated ageing and inflammatory skin diseases. Mitochondrial glutathione (mGSH) is the main line of defense for the maintenance of the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunction and cell death. [24] Glutathione can be increased via supplementation via precursors cysteine or N-acetylcysteine (not recommended for pregnant women) or the reduced form of Glutathione itself, or increased via topical active ingredients like Licochalcone A. [25] Nicotinamide NR nicotinamide ribosome which is the precursor of NMN nicotinamide mononucleotide which is the precursor of NAD+ nicotinamide adenine dinucleotide all could have a protective effect on mitochondria. Nicotinamide adenine dinucleotide is present in living organisms as ions NAD+ and NADP+ and in reduced forms NADH and NADPH. NADH is a cofactor of processes inside mitochondria:
Resveratrol Although systemically Resveratrol promotes mitochondrial biogenesis. [27] Other data shows that UVA (14 J/cm(2)) along with resveratrol causes massive oxidative stress in mitochondria. As a consequence of oxidative stress, the mitochondrial membrane potential decreases which results in opening of the mitochondrial pores ultimately leading to apoptosis in human keratinocytes. [28] Red light therapy By incorporating red light therapy into your skin care routine, you can help to counteract the damaging effects of mitochondrial dysfunction and support the skin's natural renewal processes. Next to the use of sunscreens (especially when containing Licochalcone A), CoQ10, anti-oxidants and Nicotinamide, emerging treatments like mitochondrial transfer and therapies focused on improving mitochondrial quality control processes are being investigated as potential solutions for preventing and addressing mitochondrial dysfunction in the skin. As we continue to explore the 12 hallmarks of ageing skin, I am confident that we will gain valuable insights and develop breakthrough innovations that will improve skin quality, health, beauty and vitality. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Many people associate a tan with health, beauty and an active lifestyle. Although a moderate dose of solar radiation is indispensable for our health, unfortunately, there is no such thing as a real "healthy tan" or "healthy sun-kissed glow" as it is always a visible sign of skin damage. Tanning is a response by the skin to exposure to ultraviolet (UV) radiation (and HEV or Blue Light), either from natural sunlight or artificial sources like tanning beds which leads to photo-ageing, pigmentary disorders (like age spots or hyperpigmentation) and immunosuppression, hence skin cancer. When skin is exposed to sunlight: UV rays and high energy visible light (HEV) or also called Blue Light (the most energetic region of HEV), it produces more melanin, a pigment that darkens the skin as a (partial) protective mechanism to prevent further damage. The amount of artificial blue light emitted during the conventional use of electronic devices is not enough to trigger harmful skin effects. (Click here to read more)
MELANIN Melanin is only produced by cells called melanocytes, mostly distributed in the epidermal-dermal junction. Melanocytes contain specialized organelles called melanosomes to store and produce melanin. Melanosomes are transferred from the melanocytes to the neighboring keratinocytes, which are the most abundant cells in the epidermis. One melanin-forming melanocyte surrounded by 36 keratinocytes and a Langerhans cell is called the melano-epidermal unit. [1.2] Melanocytes use the amino acid tyrosine to produce melanin and protect epidermal keratinocytes and dermal fibroblasts from the damaging effects of solar radiation.. [13] The are two melanin pigment classes:
Differences in skin pigmentation do not result from differences in the number of melanocytes in the skin, as one might assume, but from differences in the melanogenic activity (melano-competence), the type of melanin produced in melanosomes (the ratio between eumelanin and pheomelanin differs per Fitzpatrick phototype) and the size, number and packaging of melanosomes, with melanin content of melanosomes ranging from 17.9% to 72.3%. [7] The amount of melanin is never enough for adequate photoprotection, and a "base tan" does not prevent sunburn. Particularly darker phototypes are more sensitive for the damaging effects of Blue Light. Both eumelanin and pheomelanin production are promoted by UV radiation and Blue Light and therefore sunscreens offering a combination of both UV (A + B) protection and Blue Light defense are recommended for all phototypes. TANNING PROCESS The skin's tanning process occurs in four distinct phases: [3]
ROLE OF UVA, UVB AND BLUE LIGHT One of the most important acute effects of UVR is DNA photodamage. UVA and UVB show different properties regarding their biological effects on the skin. [7] Shorter wavelengths (nm) correspond to higher energy. Infrared does not induce oxidative stress. Read more UVA radiation (320-400 nm) penetrates deeper into the skin and can induce indirect DNA damage by the generation of reactive oxygen species (ROS), leading to premature skin aging. UVA, in contrast to UVB is not filtered by window glass, is able to penetrate deeper into the skin and reach the dermis. They are present constantly, with relatively equal intensity, during all daylight hours throughout the year. It has been estimated that 50% of exposure to UVA occurs in the shade. UVA rays are less intense than UVB, but there are 30 to 50 times more of them. To produce the same erythemal response, approximately 1000 times more UVA dose is needed compared with UVB. [7] The bulbs used in tanning beds emit mostly UVA. UVB radiation (280-320 nm) is less prevalent than UVA, primarily affects the outermost layers of the skin, causing direct DNA damage (more potent than UVA) and triggers inflammatory responses that lead to increased melanin production. UVB radiation fluctuates throughout the day, are at their strongest at noon. and are more cytotoxic and mutagenic than UVA. The action spectrum for UV-induced tanning and erythema are almost identical, but UVA is more efficient in inducing tanning whereas UVB is more efficient in inducing erythema (redness). Dark skin is twice as effective compared to light skin in inhibiting UVB radiation penetration. [7] UVB helps the skin to produce Vitamin D. Blue light (400-500 nm) visible light accounts for 50% of sunlight [11] and can contribute to immediate, delayed, continuous and long-lasting pigmentation by activating melanocyte-specific photoreceptors and increasing melanin synthesis, particularly in individuals with darker (melano-competent) skin types [9], cause DNA damage [10] and generate damaging reactive oxygen species in both the epidermis and the dermis. [12] The effects may last longer than those induced by UVA and UVB radiation. Blue Light can penetrate even deeper than UVA and reach the hypodermis. Blue light therapy is used to target acne causing bacteria and inflammation, however the risks might outweigh the benefits especially in darker phototypes and it might worsen acne marks. EPIDERMIS AND DERMIS Both dermal fibroblasts and epidermal keratinocytes play a crucial role in regulating skin pigmentation and tanning response. [13 15] In comparison to epidermal tanning, dermal tanning is less visible, however more immediate. Dermal fibroblasts secrete various paracrine factors that regulate melanocyte function, survival, and melanin production. Factors like hepatocyte growth factor (HGF), nerve growth factor (NGF), stem cell factor (SCF), and basic fibroblast growth factor (bFGF) stimulate melanogenesis and pigmentation [14 15] Fibroblast senescence and altered secretory profiles in conditions like melasma contribute to abnormal pigmentation by stimulating melanogenesis. [15] Epidermal keratinocytes produce factors like α-melanocyte stimulating hormone (α-MSH) and Wnt1 that activate melanogenic pathways in melanocytes, leading to increased melanin synthesis and transfer to keratinocytes. [15 16]. Keratinocyte-derived exosomes can enhance melanin production by melanocytes. [16] Differences in autophagic activity between various keratinocytes also influences pigmentation. [15] Enjoy the sun, however protect your (and your children's) skin from a photo-damaging tan to remain skin health and beauty. Sunless self-tanning products containing dihydroxyacetone (DHA) or Erythrulose provide a safe alternative to achieve a "sun-kissed" glow. You can use after-sun skin care which helps to rehydrate, reduce damage of "sun-stressed" skin and support it's repair. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

If you've scrolled through Instagram, you may have caught a glimpse of dermatologists raving about LED masks emitting red light, the secret, evidenced based weapon behind skin rejuvenation known as photo biomodulation. It uses low-powered light within the red to near-infrared range (wavelengths from 632 to 1064 nm) to induce a biological reaction aka stimulate cellular processes. The wonders of red light, also known as LLLT (low-level laser therapy), PBM (red light photo-biomodulation), or PBMT (photo-biomodulating therapy), extend far beyond non-invasive skin rejuvenation. I am not a fan of devices for home use, mostly because of lacking safety and/or efficacy, PBM definitely earned it's prominent spot in my skincare routine.
A summary of the benefts of red light with and without near infrared light for skin Numerous studies have demonstrated the effectiveness of red and infrared light therapy for skin rejuvenation. A combination of red light and near IR light has proven to stimulate the production of collagen (I & III) plus elastin production (Li WH et al Int J Cosmet Sci 2021), enhance mitochondrial ATP production, cell signaling, growth factor synthesis, rebalance ROS (reactive oxidative species) and reduce inflammation. Stem cells can be activated allowing tissue repair and healing. Wrinkle and scar reduction was observed and it can reduce UV damage both as treatment and prophylactic measure. In pigmentary disorders such as vitiligo, it can increase pigmentation by melanocyte proliferation and reduce depigmentation by inhibiting autoimmunity (Pinar Avci et al. Semin Cutan Med Surg. 2013 & Mitchell J Winkie et al. Review Photodermatol Photoimmunol Photomed A focused review of visible light therapies for vitiligo 2024). It has the potential to activate both keratinocytes (epidermis) and fibroblasts (epidermal junction and dermis). With consistent use, you can expect a reduction of lines and wrinkles, improvement of skin tone and texture. PBMT (when done effective and safe) will compliment both your skin rejuvenating and regenerating at home skincare regimen and in-office procedures or even post-surgical skin recovery. ATP ATP (adenosine triphosphate) is the primary source of energy for cellular processes and plays a crucial role in various biological functions. When red light with specific wavelengths (630 nm to 638 nm and 810 nm) is absorbed by the skin cells, it stimulates the mitochondria, which are the powerhouses of the cells responsible for ATP synthesis. This increase in ATP production is providing cells with more energy to carry out their functions effectively and has several beneficial effects on the skin like boosting cellular metabolism, promoting more efficient nutrient uptake and waste removal. The increased ATP levels facilitate collagen synthesis by fibroblasts, a vital component for skin structure, elasticity and firmness and reduction of lines and wrinkles.. ATP aids in the repair and regeneration of damaged skin cells. It accelerates the healing process, making it beneficial for wound healing, post-surgical recovery, and addressing skin issues such as acne scars. ROS (Reactive Oxidative Species) By modulating ROS levels, red light therapy helps reduce oxidative stress and its detrimental effects on the skin. ROS are highly reactive molecules that are naturally produced by cells as byproducts of metabolic processes. While low levels of ROS play important roles in cellular signaling and immune responses, excessive ROS can lead to oxidative stress and damage to cells and tissues. Restoring the balance of ROS result in improved skin health, reduced inflammation, and enhanced skin rejuvenation. Red light therapy has been shown to modulate reactive oxidative species (ROS) levels in the skin by promoting antioxidant defense mechanisms and reducing oxidative stress:
The difference between LLLT and PBM LLLT refers specifically to the use of lasers, which produce coherent, focussed and an intense beam of monochromatic light, while PBM has a broader range of light sources, may include laser as well as light-emitting diodes (LEDs) and other non-laser devices. LEDs are often used in PBM because they are cost effective, versatile and have the ability to cover large treatment areas. LLT uses higher power densities with more energy and has a shorter treatment duration in comparison to PBM to achieve desired therapeutic effects. While there are similarities in terms of mode of action", there is a difference of light source, treatment application and parameters. Based on consensus, PBM and PBMT are considered the correct way to describe this photonic specialty for therapeutic applications. In this post I will focus on PBM and specifically LEDs. LED masks and LED panels LED masks specifically produced by the brand Omnilux (FDA cleared) are currently very popular for very good reasons; they are safe and effective when the LEDs emit the right wavelengths and used in the recommended frequency. Omnilux combines 2 therapeutically effective and complimentary wavelengths: 633nm and near-infrared 830 nm. Both wavelengths (more precise 630nm + 850nm) I would recommend to minimally look for in any red LED device, which will disqualify most LED masks and panels in the market! I've include some (not affiliated) links to devices below. Both masks and panels can be effective, however most panels are stronger in comparison to masks 60 mW/cm² vs mW/cm²), hence have the benefit of a shorter treatment time to get a similar result. Intensity and power of red light therapy devices are typically measured in terms of irradiance (measured in milliwatts per square centimeter, mW/cm²) and radiant flux (measured in watts, W), which quantify the amount of light energy emitted by the device. Wearing a mask during a hot summer or in a warmer climate will make you sweat and depending on the materials of the mask and straps, they may be very uncomfortable to wear. Panels have the benefit that they give a more even distribution of emitted light as masks are worn on the face and thus the LED bulbs are pushed on a small skin surface area, panels can cover a larger area (depending on their size) and are more versatile in use, as area's like neck, décolletage, or knees are easier to treat with a panel. With a mask you may be more mobile, although I would not recommend walking around while using the mask. My personal preference would be a panel for the reasons mentioned before and panels are more suitable (more hygienic) for family sharing. My son can use it after an intense workout to speed up his recovery and I like to use it for purposes beyond photo-biomodulation or skin rejuvenation, for example to improve my sleep. With a panel I get more "bang for my buck". 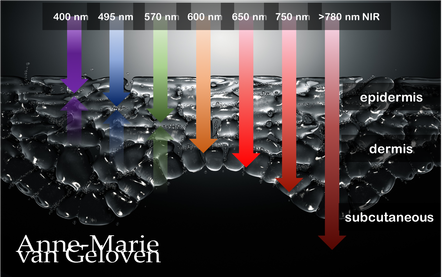
Red light and NIR (Near Infra Red light) have the ability to penetrate varying depths of the skin, resulting in distinct benefits, thus combinations of wavelengths will provide complementary effects.
630 nm Wavelength This wavelength is often used for its skin rejuvenation benefits. It has a relatively shallow penetration depth and is absorbed closer to the surface of the skin primarily affecting the epidermis. 630nm light is associated with increased circulation, reduce inflammation, improved skin tone & texture, aiding in the delivery of nutrients and oxygen to skin cells, and stimulating the production of collagen, leading to improved skin elasticity and a reduction of the appearance of fine lines & wrinkles. 660 nm Wavelength At 660nm, red light can penetrate a little deeper into the skin, reaching the dermis. It is known for its ability to stimulate collagen production, enhance cellular metabolism, and promote anti-inflammatory effects, helping to reduce redness and inflammageing. It also promotes wound healing, making it beneficial for post-surgical or post-trauma skin recovery. 810 nm Wavelength Improve healing & recovery & accelerate wound healing. 830 nm Wavelength Accelerate healing, reduce infection, improve aesthetic outcome following plastic surgery, increase endorfines (mood-enhancing), improve bone repair and growth. 850 nm Wavelength Improve general inflammation body, enhance muscle recovery, improve wound healing, reduced fine lines, wrinkles and hyperpigmentation. Always consult a qualified healthcare professional or dermatologist to determine if and what the most suitable red light therapy approach is for your particular skin condition and rejuvenation goals. Take care! References: Hamblin, Michael R. "Mechanisms and applications of the anti-inflammatory effects of photobiomodulation." AIMS biophysics 4.3 (2017): 337-361. Barolet, Daniel. Regulation of Skin Collagen Metabolism In Vitro Using a Pulsed 660 nm LED Light Source: Clinical Correlation with a Single-Blinded August 2009Journal of Investigative Dermatology 129(12):2751-9 Wunsch A, Matuschka K. (2014). A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Journal of Cosmetic and Laser Therapy, 16(5), 232-237. Avci P, et al. (2013). Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Seminars in Cutaneous Medicine and Surgery, 32(1), 41-52. Links to some devices which combine 630 nm and 850 nm: FDA-approved devices ensure safety and regulatory compliance, however the panels are more powerful: Omnilux(tm) Mask (FDA clearance) Very affordable panel (no FDA clearance) Affordable panel (no FDA clearance) |
CategoriesAll Acne Ageing Aquatic Wrinkles Armpits Biostimulators Blue Light & HEVIS Cleansing CoQ10 Cosmetic Intolerance Syndrome Deodorant Dermaplaning Diabetes Dry Skin Evidence Based Skin Care Exfoliation Exosomes Eyes Face Or Feet? Facial Oils Fibroblast Fingertip Units Gendered Ageism Glycation Gua Sha Hair Removal Healthy Skin Heat Shock Proteins Hormesis Humidity Hyaluron Hyaluronidase Hypo-allergenic Indulging Jade Roller Licochalcone A Luxury Skin Care Lymphatic Vessel Ageing Malar Oedema Menopause Mitochondrial Dysfunction Mood Boosting Skin Care Neurocosmetics Ox Inflammageing PH Balance Skin Photo Biomodulation Polynucleotides Psoriasis Regenerative Treatments Review Safety Scarring Sensitive Skin Skin Care Regimen Skin Flooding Skin Hydration Skin Senescence Skip-Care Sleep Slugging Sunscreen Tanning Under Eye Bags Vitamin C Well Ageing Skin Care Wound Healing Wrinkles
Archives
April 2024
|


 RSS Feed
RSS Feed
