
Mitochondria are the "powerhouses" or "lungs" of our cells and bioenergetic semi-autonomous organelles with their own genomes and genetic systems. [1] They are responsible for generating the energy that fuels a wide range of cellular processes in the skin, including cell signaling, pigmentation, wound healing, barrier integrity [2], metabolism and quality control. [3] Mitochondria exist in each cell of the body. Their primary role is cellular respiration; a process converting the energy in nutrients (like glucose) into a usable form of energy called ATP or Adenosine Triphosphate. Mitochondria are particularly abundant in the skin, reflecting the skin's high metabolic demand. When the functionality of mitochondria is impaired or declines, it impacts skin's vitality, health and beauty. Mitochondrial dysfunction is 1 of the 12 hallmarks of skin ageing.
The skin is particularly susceptible to mitochondrial stress due to its constant exposure to environmental insults, such as UV radiation, pollution, and other oxidative stressors. These factors can damage mitochondrial DNA, leading to increased production of reactive oxygen species (ROS) and disrupting the delicate balance of cellular processes. [4] In aged post-mitotic cells, heavily lipofuscin-loaded lysosomes perform poorly, resulting in the enhanced accumulation of defective mitochondria, which in turn produce more reactive oxygen species causing additional damage (the mitochondrial-lysosomal axis theory). [5] Optimal mitochondrial function is indispensable for sustaining the specialized functions of each cell type, like keratinocyte differentiation, fibroblast ECM production, melanocytes melanin production and distribution, immune cell surveillance, sebocytes and adipocytes. [6] Mitochondrial dysfunction is both directly and indirectly linked to chronological ageing and photo-ageing. [7] As mitochondrial function declines, the skin's ability to regenerate and repair itself is decreased. [2=1] This results in visible signs of aging, such as wrinkles, loss of elasticity, dryness, uneven pigmentation, melasma, age spots, lipomas, impaired wound healing. [2-4-5-8-9] Mitochondrial dysfunction also has been implicated in skin conditions like acne, eczema, lupus, psoriasis, vitiligo, atopic dermatitis and even skin cancer. [10] Ageing is associated with changes in mitochondrial morphology, including [6]
Good mitochondrial function or metabolism: [7]
Dysfunctional Mitochondria: [7]
Mitochondrial proteins Mitochondria contain >1,100 different proteins (MitoCoP) that often assemble into complexes and supercomplexes such as respiratory complexes and preprotein translocases. The chaperones Heat Shock Proteins HSP60-HSP10 are the most abundant mitochondrial proteins. [3] Small heat shock proteins form a chaperone system that operates in the mitochondrial intermembrane space. Depletion of small heat shock proteins leads to mitochondrial swelling and reduced respiration. [14] Mitochondrial hyperpigmentation Emerging research has shed light on the intricate relationship between mitochondrial dysfunction and the development of hyperpigmentation, a condition characterized by the overproduction and uneven distribution of melanin in the skin. One of the key mechanisms underlying this connection is the role of mitochondria in the regulation of melanogenesis, the process by which melanin is synthesized. Mitochondria are involved in the production of various cofactors and signaling molecules that are essential for the activity of tyrosinase, the rate-limiting enzyme in melanin synthesis. [15] When mitochondrial function is impaired, it can lead to an imbalance in the production and distribution of these cofactors and signaling molecules, ultimately resulting in the overproduction and uneven deposition of melanin in the skin. [15] This can manifest itself as age spots, melasma, and other forms of hyperpigmentation. The link between mitochondrial dysfunction and hyperpigmentation has been further supported by studies on genetic disorders that involve mitochondrial dysfunction, such as mitochondrial DNA depletion syndrome. In these conditions, patients often exhibit a range of pigmentary skin changes, including patchy hyper- and hypopigmentation, as well as reticular pigmentation. [16] Mitochondrial crosstalk and exosomes Mitochondria can crosstalk and move beyond cell boundaries. [17] Mitochondria-derived material might be transferred to neighboring cells in the form of cell-free mitochondria or included in extracellular vesicles [18-19]. This process supports cellular repair and contributes to vital mitochondrial functions. Besides restoring stressed cells and damaged tissues due to mitochondrial dysfunction, intercellular mitochondrial transfer also occurs under physiological and pathological conditions. [20] The transfer of active mitochondria from mesenchymal stem cells (MSCs) has been identified as a repair mechanism for rejuvenating damaged skin fibroblasts. [21] MITOCHONDRIAL SUPPORT Q10 or Coenzyme Q10 (CoQ10) Q10 is part of the mitochondrial respiration chain and essential for cellular energy production. About 95% of our cellular energy is generated with support of Q10, which is produced by the human body itself. During skin ageing, both the cellular energy production and levels of Q10 are declined. Q10 is a powerful anti-oxidant [22], thus protecting cells from oxidative stress and damage and has proven to be able to "rescue" senescent cells by decreasing elevated senescent markers like p21 levels and β-Galactosidases positive cell numbers (in-vitro). Q10 is bio-active, increasing collagen type I and elastin production. [23=8] Q10 can be supplemented via nutrition, however also via topical application and is considered an evidence based active ingredient in skin care products. Ubiquinol (reduced form) shows higher bioavailability compared to ubiquinone (oxidized form). [23] Glutathione Glutathione is formed in cell's cytoplasm from glutamic acid, cysteine and glycine. It is present in 2 forms: reduced (GSH) and oxidized (GSSG). Reduced GSH is an active anti-oxidant, while the presence of inactive GSSG is increased under oxidative stress. The ratio between GSH and GSSH is considered a measure of oxidative stress. Glutathione participates in redox reactions, acts as co-factor of many anti-oxidant enzymes and is the most important non-enzymatic anti-oxidant, essential for synthesis of proteins and DNA. Low Glutathione results in accelerated ageing and inflammatory skin diseases. Mitochondrial glutathione (mGSH) is the main line of defense for the maintenance of the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunction and cell death. [24] Glutathione can be increased via supplementation via precursors cysteine or N-acetylcysteine (not recommended for pregnant women) or the reduced form of Glutathione itself, or increased via topical active ingredients like Licochalcone A. [25] Nicotinamide NR nicotinamide ribosome which is the precursor of NMN nicotinamide mononucleotide which is the precursor of NAD+ nicotinamide adenine dinucleotide all could have a protective effect on mitochondria. Nicotinamide adenine dinucleotide is present in living organisms as ions NAD+ and NADP+ and in reduced forms NADH and NADPH. NADH is a cofactor of processes inside mitochondria:
Resveratrol Although systemically Resveratrol promotes mitochondrial biogenesis. [27] Other data shows that UVA (14 J/cm(2)) along with resveratrol causes massive oxidative stress in mitochondria. As a consequence of oxidative stress, the mitochondrial membrane potential decreases which results in opening of the mitochondrial pores ultimately leading to apoptosis in human keratinocytes. [28] Red light therapy By incorporating red light therapy into your skin care routine, you can help to counteract the damaging effects of mitochondrial dysfunction and support the skin's natural renewal processes. Next to the use of sunscreens (especially when containing Licochalcone A), CoQ10, anti-oxidants and Nicotinamide, emerging treatments like mitochondrial transfer and therapies focused on improving mitochondrial quality control processes are being investigated as potential solutions for preventing and addressing mitochondrial dysfunction in the skin. As we continue to explore the 12 hallmarks of ageing skin, I am confident that we will gain valuable insights and develop breakthrough innovations that will improve skin quality, health, beauty and vitality. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References
Comments

In skin biology, senescence is a process by which a cell ages and permanently stops dividing but does not die. This is why they are also referred to as "zombie cells". Age-related accumulation of senescent cells is caused by of increased levels of senescence-inducing stressors and/or reduced elimination of senescent cells. Under normal physiological conditions, senescent cells play an important role maintaining cellular homeostasis and inhibiting proliferation of abnormal cells. However, over time, large numbers of zombie cells can build up in the skin and contribute to the overall reduction in skin's regenerative properties, impacting both its beauty and health.
There are 2 forms of cell senescence: Acute senescence: Senescent cells are produced in response to acute stressors to facilitate for example tissue repair, wound healing. They are cleared by our immune system. Chronic senescence: A not programmed process as response to prolonged stress or damage and these senescent cells are not cleared by our immune system, leading to the accumulation of zombie cells impacting our skin health and beauty. It has been suggested that inflammageing is mainly related to senescent cells and their associated SASP (Senescence-Associated Secretory Phenotype) which increase in the body with age and contribute to inflammageing. Senescent cells cause inflammageing and inflammageing causes cell senescence. [1] Senescence can be triggered by a number of stress signals to the cell [1]:
Mechanisms of skin cell senescence:
The presence of senescent cells accelerates the ageing process due to their communication with nearby cells through various molecules: [18]
Fibroblast senescence could be the main driver of the skin ageing. [3] The increased number of senescent fibroblasts results in the production of SASPs rich in pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-8, IL-18, matrix metalloproteinases (MMPs), and a variety of other inflammatory chemokines [2] resulting in the breakdown of collagen, loss of elasticity and wrinkle formation. [3] Autophagy in dermal fibroblasts is essential for maintaining skin balance and managing the ageing process, particularly in response to external stressors like UV radiation and particulate matter (PM), by repairing cellular machineries. [4] Insufficient autophagy leads to an exaggerated skin inflammation triggered by inflammasome activation, resulting in accelerated ageing characteristics. When exposed to UVB (in vitro), skin cell types like fibroblasts and keratinocytes show DNA damage and increased senescence markers, such as increased SASPs. [3] Dermal fibroblasts also release insulin-like growth factor (IGF)-1, essential for epidermal cell proliferation and differentiation. [5] IGF-1 signalling in senescent fibroblasts is significantly decreased [6]. Inhibition of the IGF-1 pathway decreases collagen production in the dermis, causing epidermal thinning. Additionally, mitochondrial dysfunction and increased levels of superoxide anions prompt fibroblast ageing, thereby speeding up the skin ageing process. [5] Fibroblasts isolated from photo-aged skin produce a greater amount of pro-melanogenic growth factors. [14] Ageing-associated pigmentation has also been reported to be driven by (UVA-induced) fibroblast senescence. [15-16] Keratinocyte senescence The epidermis shows less impact of senescent keratinocytes due to their quicker turnover in comparison to fibroblasts. Senescent keratinocytes experience reduced ECM production and cell adhesions [8], along with elevated MMP expression in UV-induced senescence [9], and increased SASP levels, including pro-inflammatory cytokines. [10] Airborn particulate matter (PM2.5) can penetrate a disrupted skin barrier. PM2.5-induced ROS leads to epigenetic modification: reduced DNA methyltransferase, elevated DNA demethylase expression, p16INK4a promotor hypomethylation and therewith accelerated keratinocyte senescence. [11] Keratinocytes are the main type of cells that signal the need for melanogenesis. [12] UVR-induced DNA damage in keratinocytes activates melanogenesis. [13] Melanocyte senescence Senescent melanocytes express markers of inflammageing and dysfunctional telomeres. Senescent melanocyte SASPs induce telomere dysfunction and limit the proliferation of the surrounding cells, hence, senescent melanocytes affect and impair basal keratinocyte proliferation and contribute to epidermal atrophy. [17] STRATEGIES TO COMBAT CELL SENESCENCE PREVENTION Sunscreen: Protection against UV radiation combined with blue light defense (Licochalcone A: powerful anti-oxidant, Nrf2-Activator & increasing Glutathione + Colour pigments) and prevention + repair DNA damage (Glycyrrhetinic Acid) INTERVENTION Senotherapeutics can be classified into three development strategies: [25]
Skin care ingredients: [18]
Of course a healthy life-style and diet (consider also intermittent fasting) will support both your body & skin longevity and beauty Prevention and intervention of skin cell senescence offers a promising approach to improve skin health and beauty. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Many of the skin regenerating or rejuvenating treatments, like energy based devices in the doctors-office are based on the principle to cause controlled damage and therewith provocation of a skin rejuvenating repair response. One of the fascinating mechanisms behind laser "damage" is the heat shock response leading to increased production of regenerating heat shock proteins (HSPs). Heat shock proteins respond to heat stress, are crucial cellular defense mechanisms against stress (environmental and physiological), act as chaperones, aiding in protein folding, prevention of protein damage, cellular protection and repair. [1]
HEAT SHOCK PROTEINS AND OX-INFLAMMAGEING UV radiation and blue light cause oxidative stress and inflammation, and can overwhelm skin's own capacity to counteract the increased formation of reactive oxygen species (ROS) and inflammatory mediators. Chronic oxidative stress state and chronic low grade of inflammation are hallmarks of skin ageing and their combination can be called ox-inflammageing. Oxidative stress and inflammation alter cellular signal transduction pathways and thereby the expression of the ECM genes as well as the structure of the ECM proteins like collagen, fibronectin and elastin. Their reduced expression and increased degradation manifests eventually at the skin surface as wrinkles, loss of firmness, and elasticity. Heat shock proteins are chaperone proteins that facilitate the formation of the ECM and prevention of molecular oxidative damage or degradation and are classified based on their molecular weights.
STIMULATION OF REJUVENATING HEAT SHOCK PROTEINS Heat shock protein synthesis can be initiated not only by heat but also by many chemical and physical stimuli, such as heavy metals, amino acid analogues, oxidative stress, viral infection and UV and ionizing irradiation. [10] Laser Laser treatments have been shown to induce a heat shock response in the skin from epithelial cells to deeper connective tissues, leading to the production of heat shock proteins. This response is characterized by the temporary changes in cellular metabolism, release of growth factors, and increased cell proliferation and thus contribute to tissue regeneration and rejuvenation. [11] CBD It has been proven that a large number of genes belonging to the heat shock protein super-family were up-regulated following cannabidiol (CBD) treatment. [12] UV radiation Ultraviolet radiation (UV)‐induced cell death and sunburn cell formation can be inhibited by previous heat shock exposure and UV itself can induce HSP expression. However, levels of HSP-27 have been found to be elevated in sun‐protected aged skin indicating a link between HSP-27 expression and age‐dependent epidermal alterations. [13] I would recommend daily protection from UV radiation and blue light (or high energy visible light). Ultrasound Ultrasound exposure at different frequencies, intensities, and exposure times can induce HSP-72 expression. Higher ultrasound frequencies, such as 10 MHz, have been found to significantly increase HSP-72 levels. Additionally, increasing the temperature during ultrasound exposure has shown to enhance HSP-72 expression. Interestingly, ultrasound at 1 MHz was unable to induce HSP-72 significantly, while 10 MHz ultrasound induced HSP-72 after 5 minutes of exposure. [10] Radiofrequency Radiofrequency has been shown to increase HSP-70 and decrease melanin synthesis and tyrosinase activity. [14] RF-US treatment significantly increased levels of HSP47 proteins. [15] Red & near infra red light Although I've not seen much peer reviewed published evidence, red light and near infra red light therapy may release the HSPs in the skin if tissue reaches >42 - 45 degrees (even for 8 - 10 seconds). Nicotinamide Nicotinamide and its derivatives have been found to stimulate the expression of heat shock proteins, including HSP-27, HSP-47, HSP-70, and HSP-90 in the skin. These proteins play as mentioned before an essential role in collagen production, skin protection, skin health and rejuvenation. [6] NAD as nutrient interestingly has proven to tweak the epigenome by modulating DNMT1 enzymatic DNA methylation and cell differentiation. [16] In topical applications an ingredient called Dihydromyricetin also called Epicelline® has been successful in inhibiting DNMT1 enzyme activity biochemical assays. [17] Stimulation of heat shock proteins offers a promising and novel invasive, non invasive and topical approach for skin regeneration, rejuvenation and reduction of ox-inflammageing. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Like epigenetics and exosomes, neurocosmetics represent a revolutionary approach for skin care incorporating neuroscience principles, leveraging the skin-brain connection to improve skin health and beauty. The term itself is a fusion of the words neuroscience and cosmetics. It differs from psychodermatology which like neurocosmetics connects the interaction between mind and skin, but in a different way. Some describe it as how simple sensory stimulation can improve our overall wellbeing and call it "mood beauty", however this doesn't do it justice as neurocosmetics go beyond mood boosting skincare.
DEFINITION NEUROCOSMETICS Dermatologist Professor Laurent Misery back in 2002 described that neurocosmetics are products which are supposed to modulate the neuro-immuno-cutaneous-system (NICS) function at an epidermal level. Skin cells can produce neuromediators, which are mediators for transmission of information between skin, immune and the nervous system. All skin cells express specific receptors for neuromediators and by binding of the neuromediator to its receptor, modulation of cell properties and skin functions are induced like cell differentiation and proliferation (renewal), pigmentation, etc. Hence, keratinocytes, Langerhans cells, melanocytes, endothelial cells, fibroblasts and the other cells of the skin are modulated and controlled by the nerves and in return skin is able to modulate neuronal activity and growth. [1] SKIN-BRAIN CONNECTION In an article from the International Journal of Novel Research and Developments, the skin-brain connection was described as a psychobiological concept that highlights how emotions, stress, and neurotransmitters impact skin health. Indicating that the skin acts as a neuroimmunoendocrine organ, emphasizing its sensitivity to neural signals and stress responses. [4] CUTANEOUS NERVOUS SYSTEM The skin a sophisticated sensory organ that allows you to interact with your environment through touch and feel. It contains a complex network of nerves that send information about sensations like pressure, pain, itch and temperature from the skin through the spinal cord to the brain [9]. The dynamic interactions between the skin and the nervous system is influenced by factors like stress and inflammation, which can impact skin health and ageing. [7] Nerves in the skin: These nerves are like tiny messengers that tell your brain about what your skin is feeling: pressure, heat or pain. Types of nerve fibers: Some are thick and wrapped in a protective coating, which helps them send messages quickly. Others are thin and slow but are very good at sending messages about pain or temperature changes. [3] Sensory receptors: These receptors can tell if something is touching the skin lightly or if there's a lot of pressure. They can also sense if something is hot, cold, or causing pain. [3] Autonomic nervous system: Part of the cutaneous nervous system helps control things that happen in the skin automatically, like sweating to regulate body temperature. [8] Nerve cells: There are about 20 different types of neurons in our skin. [10] The contribution of epidermal keratinocytes to NICS [3]
CUTANEOUS NEURO-AGEING Neuro-ageing is defined as the changes in the nervous system which cause continuous neurodegeneration due to oxidative stress, neuroinflammation or impaired neuromodulation. As skin ages, Aβ-toxin (increased by oxidative stress) accumulates at the nerve endings innervating the tissue, causing disrupted cellular communication, particularly affecting fibroblasts’ ability to produce collagen and extracellular matrix. On top there is a decrease of nerve growth factor (NGF) production, important for the development and maintenance of nerve cells. Different factors can lead to a drop in NGF production, resulting in malfunctioning keratinocytes and reduced lipolytic activity of adipocytes, visibly impacting skin hydration and firmness. [6] Skin nerve fibres are significantly reduced in number following UV irradiation and in ageing skin [5] and therefore neuro-protectors or targetting neurodegeneration can reduce stress manifestations and promote healthy cellular communication for optimal skin function. [3] Although not much is known regarding skin specific or topical neuroprotectors (most research was focussed on the brain), probably potent anti-oxidants, by significantly reducing oxidative stress from UV and blue light and anti-inflammatory ingredients may inhibit skin neuro-ageing and can be neuroprotective especially when combined with sunscreen and strengthening of the skin barrier. NEUROCOSMETIC VARIETY OF ACTIONS
THE FUTURE OF NEUROCOSMETICS The neurocosmetics market is booming, with a projected value of USD 2.69 billion by 2030. [11] The future of neurocosmetics holds promise for innovative ingredients and concepts that harness new neuroscientific insights to revolutionize skin care and sunscreen formulations, to cater to both physical and emotional aspects of skin health and beauty. Take care! Anne-Marie References

If you've scrolled through Instagram, you may have caught a glimpse of dermatologists raving about LED masks emitting red light, the secret, evidenced based weapon behind skin rejuvenation known as photo biomodulation. It uses low-powered light within the red to near-infrared range (wavelengths from 632 to 1064 nm) to induce a biological reaction aka stimulate cellular processes. The wonders of red light, also known as LLLT (low-level laser therapy), PBM (red light photo-biomodulation), or PBMT (photo-biomodulating therapy), extend far beyond non-invasive skin rejuvenation. I am not a fan of devices for home use, mostly because of lacking safety and/or efficacy, PBM definitely earned it's prominent spot in my skincare routine.
A summary of the benefts of red light with and without near infrared light for skin Numerous studies have demonstrated the effectiveness of red and infrared light therapy for skin rejuvenation. A combination of red light and near IR light has proven to stimulate the production of collagen (I & III) plus elastin production (Li WH et al Int J Cosmet Sci 2021), enhance mitochondrial ATP production, cell signaling, growth factor synthesis, rebalance ROS (reactive oxidative species) and reduce inflammation. Stem cells can be activated allowing tissue repair and healing. Wrinkle and scar reduction was observed and it can reduce UV damage both as treatment and prophylactic measure. In pigmentary disorders such as vitiligo, it can increase pigmentation by melanocyte proliferation and reduce depigmentation by inhibiting autoimmunity (Pinar Avci et al. Semin Cutan Med Surg. 2013 & Mitchell J Winkie et al. Review Photodermatol Photoimmunol Photomed A focused review of visible light therapies for vitiligo 2024). It has the potential to activate both keratinocytes (epidermis) and fibroblasts (epidermal junction and dermis). With consistent use, you can expect a reduction of lines and wrinkles, improvement of skin tone and texture. PBMT (when done effective and safe) will compliment both your skin rejuvenating and regenerating at home skincare regimen and in-office procedures or even post-surgical skin recovery. ATP ATP (adenosine triphosphate) is the primary source of energy for cellular processes and plays a crucial role in various biological functions. When red light with specific wavelengths (630 nm to 638 nm and 810 nm) is absorbed by the skin cells, it stimulates the mitochondria, which are the powerhouses of the cells responsible for ATP synthesis. This increase in ATP production is providing cells with more energy to carry out their functions effectively and has several beneficial effects on the skin like boosting cellular metabolism, promoting more efficient nutrient uptake and waste removal. The increased ATP levels facilitate collagen synthesis by fibroblasts, a vital component for skin structure, elasticity and firmness and reduction of lines and wrinkles.. ATP aids in the repair and regeneration of damaged skin cells. It accelerates the healing process, making it beneficial for wound healing, post-surgical recovery, and addressing skin issues such as acne scars. ROS (Reactive Oxidative Species) By modulating ROS levels, red light therapy helps reduce oxidative stress and its detrimental effects on the skin. ROS are highly reactive molecules that are naturally produced by cells as byproducts of metabolic processes. While low levels of ROS play important roles in cellular signaling and immune responses, excessive ROS can lead to oxidative stress and damage to cells and tissues. Restoring the balance of ROS result in improved skin health, reduced inflammation, and enhanced skin rejuvenation. Red light therapy has been shown to modulate reactive oxidative species (ROS) levels in the skin by promoting antioxidant defense mechanisms and reducing oxidative stress:
The difference between LLLT and PBM LLLT refers specifically to the use of lasers, which produce coherent, focussed and an intense beam of monochromatic light, while PBM has a broader range of light sources, may include laser as well as light-emitting diodes (LEDs) and other non-laser devices. LEDs are often used in PBM because they are cost effective, versatile and have the ability to cover large treatment areas. LLT uses higher power densities with more energy and has a shorter treatment duration in comparison to PBM to achieve desired therapeutic effects. While there are similarities in terms of mode of action", there is a difference of light source, treatment application and parameters. Based on consensus, PBM and PBMT are considered the correct way to describe this photonic specialty for therapeutic applications. In this post I will focus on PBM and specifically LEDs. LED masks and LED panels LED masks specifically produced by the brand Omnilux (FDA cleared) are currently very popular for very good reasons; they are safe and effective when the LEDs emit the right wavelengths and used in the recommended frequency. Omnilux combines 2 therapeutically effective and complimentary wavelengths: 633nm and near-infrared 830 nm. Both wavelengths (more precise 630nm + 850nm) I would recommend to minimally look for in any red LED device, which will disqualify most LED masks and panels in the market! I've include some (not affiliated) links to devices below. Both masks and panels can be effective, however most panels are stronger in comparison to masks 60 mW/cm² vs mW/cm²), hence have the benefit of a shorter treatment time to get a similar result. Intensity and power of red light therapy devices are typically measured in terms of irradiance (measured in milliwatts per square centimeter, mW/cm²) and radiant flux (measured in watts, W), which quantify the amount of light energy emitted by the device. Wearing a mask during a hot summer or in a warmer climate will make you sweat and depending on the materials of the mask and straps, they may be very uncomfortable to wear. Panels have the benefit that they give a more even distribution of emitted light as masks are worn on the face and thus the LED bulbs are pushed on a small skin surface area, panels can cover a larger area (depending on their size) and are more versatile in use, as area's like neck, décolletage, or knees are easier to treat with a panel. With a mask you may be more mobile, although I would not recommend walking around while using the mask. My personal preference would be a panel for the reasons mentioned before and panels are more suitable (more hygienic) for family sharing. My son can use it after an intense workout to speed up his recovery and I like to use it for purposes beyond photo-biomodulation or skin rejuvenation, for example to improve my sleep. With a panel I get more "bang for my buck". 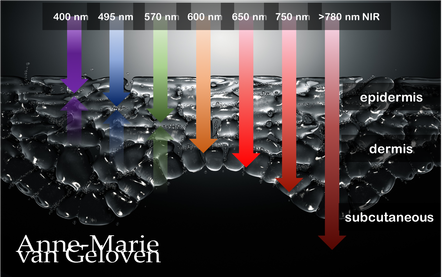
Red light and NIR (Near Infra Red light) have the ability to penetrate varying depths of the skin, resulting in distinct benefits, thus combinations of wavelengths will provide complementary effects.
630 nm Wavelength This wavelength is often used for its skin rejuvenation benefits. It has a relatively shallow penetration depth and is absorbed closer to the surface of the skin primarily affecting the epidermis. 630nm light is associated with increased circulation, reduce inflammation, improved skin tone & texture, aiding in the delivery of nutrients and oxygen to skin cells, and stimulating the production of collagen, leading to improved skin elasticity and a reduction of the appearance of fine lines & wrinkles. 660 nm Wavelength At 660nm, red light can penetrate a little deeper into the skin, reaching the dermis. It is known for its ability to stimulate collagen production, enhance cellular metabolism, and promote anti-inflammatory effects, helping to reduce redness and inflammageing. It also promotes wound healing, making it beneficial for post-surgical or post-trauma skin recovery. 810 nm Wavelength Improve healing & recovery & accelerate wound healing. 830 nm Wavelength Accelerate healing, reduce infection, improve aesthetic outcome following plastic surgery, increase endorfines (mood-enhancing), improve bone repair and growth. 850 nm Wavelength Improve general inflammation body, enhance muscle recovery, improve wound healing, reduced fine lines, wrinkles and hyperpigmentation. Always consult a qualified healthcare professional or dermatologist to determine if and what the most suitable red light therapy approach is for your particular skin condition and rejuvenation goals. Take care! References: Hamblin, Michael R. "Mechanisms and applications of the anti-inflammatory effects of photobiomodulation." AIMS biophysics 4.3 (2017): 337-361. Barolet, Daniel. Regulation of Skin Collagen Metabolism In Vitro Using a Pulsed 660 nm LED Light Source: Clinical Correlation with a Single-Blinded August 2009Journal of Investigative Dermatology 129(12):2751-9 Wunsch A, Matuschka K. (2014). A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Journal of Cosmetic and Laser Therapy, 16(5), 232-237. Avci P, et al. (2013). Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Seminars in Cutaneous Medicine and Surgery, 32(1), 41-52. Links to some devices which combine 630 nm and 850 nm: FDA-approved devices ensure safety and regulatory compliance, however the panels are more powerful: Omnilux(tm) Mask (FDA clearance) Very affordable panel (no FDA clearance) Affordable panel (no FDA clearance) 
Glycation is one of the basic root causes of endogeneous (intrinsic) skin ageing and a very challenging one or almost impossible one to reverse. Glycation is an ageing reaction which begins in early life, developing clinical symptoms at around 30, and progressively accumulates in tissues and skin due to the glycated collagens that are difficult to be decomposed. Glycation occurs naturally in the body when sugars react with proteins and lipids to form advanced glycation end products (AGEs). AGEs can be exogenously ingested (through food consumption), inhaled via tobacco or endogenously produced and formed both intracellularly and extracellularly. AGE modifications lead to dermal stiffening, diminished contractile capacity of dermal fibroblasts, lack of elasticity in the connective tissues, contribute to hyperpigmentation and a yellowish skin appearance. The formation of AGEs is amplified through exogenous factors, e.g., ultraviolet radiation.
AGEs cause changes in the skin through 3 processes:
One study published in the Journal of Investigative Dermatology found that levels of AGEs were higher in the skin of older individuals compared to younger ones. The study also showed that there was a correlation between the level of AGEs and the severity of skin ageing. This suggests that inhibiting the production or accumulation of AGEs in the skin is a potential target for anti-ageing interventions or skin ageing management. AGEs are complex and heterogeneous, more than a dozen AGEs have been detected (however not all) in tissues and can be divided into three categories according to their biochemical properties. AGEs are formed through four pathways:
GLYCATION INHIBITION IS KEY AGEs can be crosslinked through side chains to form a substance of very high molecular weight, which is not easily degraded. The consequences from skin glycation are irreversible. This makes prevention or inhibition of the process the best potential strategy to maintain skin health and ageing skin management. One way to do this is by altering the diet to reduce the intake of sugars and carbohydrates, which are known to contribute to glycation. Several studies have found that reducing sugar intake can result in significant improvements in skin health, including reducing wrinkles and improving skin texture. 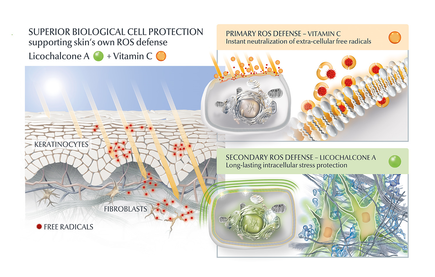
AGE inhibitors
Another potential strategy is the use of topical agents that inhibit the formation or accumulation of AGEs in the skin. One study published in the Journal of Cosmetic Science found that a cream containing carnosine, a peptide that inhibits glycation, improved skin elasticity and reduced the appearance of wrinkles in individuals with ageing skin. Skincare containing NAHP or Acetyl Hydroxyproline inhibits the formation of AGEs significantly (in vitro), most likely through a mechanism where NAHP competes with the proteins for the sugar. Finally, NAHP sacrifices itself in place of the proteins and gets (at least partially) glycated. NAHP also prevents loss of cellular contractile forces in a glycated in vitro dermis model and counteracts the diminished cell-matrix interaction that is caused by glyoxal-induced AGE formation. Anti-Oxidants Moreover, I would suggest to combine those ingredients with an ingredient like Licochalcone A. Numerous high ranked publications support that Licochalcone A protects cells from oxidative stress mediated by e.g. UV and HEVIS (blue light) induced reactive oxidative species (ROS). Due to the activation and nuclear translocation of the transcription factor NrF2, the expression of anti-inflammatory, antioxidant and detoxifying enzymes are induced. These enzymes protect the skin cells (like keratinocytes and fibroblasts) from ROS-induced damage, like lipid peroxidation and DNA as well as protein damage. If Licochalcone A is combined with L-Ascorbic Acid, (the most active form of Vitamin C), it supporting skin's own collagen production, provides superior biological cell protection amongst other relevant benefits. My absolute favourite product is Eucerin Hyaluron-Filler Vitamin C Booster which I use daily as a serum in my morning routine. GLYCATION AND SKIN HEALTH Acne In addition to its role in ageing, glycation in the skin has also been linked to a range of skin health problems. One study published in the Journal of Cosmetic Dermatology found that the level of AGEs in the skin was significantly higher in individuals with acne than in those without acne. The study also showed that treating acne with a topical antibiotic significantly reduced the levels of AGEs in the skin. Atopic Dermatitis Another study published in the Journal of Investigative Dermatology found that individuals with atopic dermatitis had higher levels of AGEs in their skin than healthy individuals. This suggests that glycation may play a role in the development of inflammatory skin conditions. Diabetes + Woundhealing The correlation between high sugar levels and skin ageing can be seen in diabetic patients, where one-third of this population has skin complications. A prominent feature of ageing human skin is the fragmentation of collagen fibers, which severely damages the structural integrity and mechanical properties of the skin. Elevated levels of MMP-1 and MMP-2 and higher crosslinked collagen in the dermis of diabetic skin lead to the accumulation of fragmented and crosslinked collagen, thereby impairing the structural integrity and mechanical properties of dermal collagen in diabetes. Collagen crosslinking makes it impossible for them to easily repair, resulting in reduced skin elasticity and wrinkles. Keratinocytes and fibroblasts are the main cells involved in wound healing, but due to the high glucose (HG) microenvironment in diabetics, the functional state of these cells is impaired, thereby accelerating cellular senescence (programmed cell death). Conclusion We can't completely stop the glycation process, therefore it's important that we inhibit it from a young age onwards, hence monitor the sugar intake of our children, use daily SPF and invest in good dermo-cosmetic products containing ingredients like NAHP and powerful anti-oxidants like L-Ascorbid Acid (Vitamin C is needed for the production of collagen) and Licochalcone A (also anti-inflammatory). Preventing signs of ageing, specifically caused by glycation is most effective. If your skin shows (advanced) signs of ageing, you can get visible improvement using skin component (hyaluron, collagen and elastin) bio-stimulating ingredients like Retinol, Bakuchiol, Arctiin, Creatine or Glycine Saponin. Consult your dermatologist if you wish to improve your skin's appearance or skin health issues. Take care Special thanks: Ph.D. dr Julia M. Weise Manager Biological Testing & Dorothea Schweiger Lab Manager Facial Skin Biology Beiersdorf HQ Hamburg 3/21/2023 Comments Exosomes in skin care and treatments
Skin boosters using micro-injections with predominantly non-crosslinked hyaluron filler gels like Restylane® Vital, Juvéderm® VOLITE or Belotero® Revive are gaining popularity for very good reasons. Unlike traditional dermal fillers, they are not injected beneath the skin to volumise or shape the face. Instead, they are very fine dermal easily integrated "fillers" that are injected into the skin to hydrate, improve skin quality and give very natural results. They are also gently bio-stimulating, meaning they "stretch" the fibroblasts in the injected area and as a result this cell is producing more collagen. An effective bio-remodeling skin booster using 2 times 5 injection points (bio-aesthetic points - BAP) for a full-face treatment is Profhilo®. However, the recent K-beauty treatment via topical application or micro-injections with bio-remodeling exosomes is gaining popularity.
Exosomes are nano-sized cargos with a lipid bilayer structure carrying diverse biomolecules including lipids, proteins, and nucleic acids. These small extra cellular vesicles are secreted by most types of cells (skin relevant are the keratinocytes and fibroblasts) to communicate with each other. Exosomes circulate through bodily fluids and can transfer information. They can be either good or bad, however taken from a healthy young cell they will be sending the best messages. Studies have shown the efficacy of exosomes in skin ageing. They can facilitate skin remodeling (increasing collagen and decreasing senescent cells) leading to skin rejuvenation. Cells sleep because they don't get enough bio-stimulation: messages. Better messages is better skin architecture. This is why exosomes are so important. At the World Stem Cell Summit it used to be 90% about stem cells (they only life 28 days) and 10% about exosomes, now it is 50/50. The reason is called heterochronic parabiosis. 1. One of the most robust methods of improving the function of ageing tissues is that of heterochronic parabiosis,. The effect was shown in a study with a surgical procedure whereby a young and old mouse are joined together so the share one circulatory system. 2 This study according to dr Kate Goldie AMWC 2023 Monaco is proof that it is not the cells, but the messages they give that is transforming lots of different tissues, which has the ability to profoundly regenerate tissues. That is why people are so interested in exosomes. Exosomes taken from a very young cell give potentially the best messages as they "send the message" of youth. EV (Extra-cellular Vesicle) is the actual correct term as messages come as micro-vesicles and exosomes and form 2 different messages from the cell. 3 We start to understand active ingredients. In exosomes one of the most important ingredients is RNA and is part of the future of regenerative aesthetics. Messenger RNAs up-regulate and Micro-RNAs down-regulate. They physically go into the cell and change how the cells works. So we have to be cautious. In this study "The therapeutic and commercial landscape of stem cell vesicles in regenerative dermatology" dr Kate Goldie et al. assessed all available exosomes in the (UK) market. Most exosomes used in-office are extracted from human stem cells and frozen to keep them as stable. Unlike actual stem cells, exosomes don't have a nucleus and therefore they are safe to use. Exosome therapy is the application of topical exosomes after in-office treatments which disrupt the skin barrier, like laser resurfacing, chemical peelings or microneedling. Exosomes are also used in micro-injections as a stand-alone skin boosting treatment and in a few skin care products. Be aware that as usual, not all products are alike. The way exosomes are sourced (origin), size, their content (can be growth factors) and function determine largely their efficacy and the price of the product. One of the challenges is that we do not really know what is in the exosomes. They are like small packages with a lot of messengers. The use of exosomes looks promising for several indications: regenerative aesthetic medicine, healing, scar treatment, burns and atopic dermatitis, however their safety is not yet fully established and no official registration for their use granted. Take care 1. Cell Cycle. 2012 Jun 15; 11(12): 2260–2267. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches Irina M. Conboy 2. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types Methodios Ximerakis 3. J Cell Biol. 2013 Feb 18; 200(4): 373–383. Extracellular vesicles: Exosomes, microvesicles, and friends Graça Raposo et al 
The fibroblast is one of the most important cells involved in ageing skin. You can find it in the lower layer of the epidermis and the dermis. It has many functions, one of which is the production of key components like hyaluron (filling + hydration), collagen (strength + structure) and elastin (flexibility + stretch). It particularly has to work hard to replenish hyaluronic acid or hyaluron as this filling component only has a half-life in the skin of several hours up to a day. Good quality collagen can last 15 years and elastin up to 70 years. It is also believed to be involved in the clean-up of dysfunctional components, like for example broken elastin, which is visible photodamage-damage and called solar elastosis. Fibroblast senescence (agedness) does also increase the risk of age spots. In proper ageing skin management, the fibroblast is a key target-cell.
Many aesthetic in-office treatments like ultrasound, radio-frequency, chemical peelings, laser etc. are based on causing controlled damage to the skin provoking wound-healing. This is the base of their rejuvenating or aesthetic impact. The number of new fibroblasts (myofibroblasts) is increased during the wound-healing process. Some injectables, like for example hyaluron-fillers cause the fibroblasts at the injection site to stretch and bio-stimulate collagen production. There are specific bio-stimulating injectable treatments. The most popular ones are Sculptra®, Radiesse®, Ellanse®, and a new one which combines hyaluron-filling and bio-stimulation is HArmonyCa®. As we age the fibroblast is undergoing some changes because of intrinsic and extrinsic factors. It loses it’s production power, it flattens, loses mechanical tension and therewith the ability to interact with other cells in the skin. It is becoming “tired and deaf”. My hypothesis was that injecting large droplets of hyaluron into the dermis might cause the fibroblast to become “lazy” via a negative feedback mechanism: when something is present in abundance, the fibroblast might not be stimulated enough to work hard to replenish it. This is not yet scientifically proven. It is important to keep the fibroblast in good shape and biologically active. We can stimulate it’s biological activity with skincare containing bio-stimulators, or ingredients which activate the production of important skin components by the fibroblast. On the other side we need to protect the cell from damage. Bio-stimulating active ingredients in skincare which have shown to particularly stimulate the fibroblast* are for example:
Protection from photo-damage we can achieve with a combination of sunscreen and anti-oxidants, more specifically Licochalcone A. Licochalcone A has a proven broad ability to protect the skin from damaging free-radicals or oxidative stress from UVA, UVB and HEVIS (High Energy Visible Light) affecting keratinocytes and fibroblasts. I am not yet aware of skincare ingredients which increase the number of (new) fibroblasts, like the semi or minimal invasive in-office treatments. It’s an interesting field to explore if this is possible without injury, inflammation or irritation. However, you probably get "more bang for your buck" by starting a a skincare routine with focus on bio-stimulation and protection of the fibroblast pre- and post minimal and semi invasive aesthetic treatments. This could be something we will proof with a clinical study. Take care *in vitro 12/15/2018 Comments Aquatic wrinkles
After about spending some time in bathtub or in the pool, we can notice that our skin on particularly finger tops and toes start to wrinkle up. This wrinkling effect is believed to have a function.
When wet, things tend to be more slippery and our sophisticated skin is designed to counteract this by wrinkling up in a pattern optimised to provide a drainage network that improves grip, much like the tires on a car according to a study. Link to original publication. However, other studies would contradict that there would be a functional benefit for so called aquatic wrinkles. The osmosis theory Water molecules moving trough a semipermeable membrane from a low concentration area to a high concentration area is a process called osmosis. The shrinking and expanding effects of osmosis takes place simultaneously outer layer of the skin, causing wrinkles. The skin's outermost layer is also known as stratum corner could be responsible for this wrinkly reaction, The top layer of our skin consists of dead corneocytes. The longer these cells are attached to the skin, the bigger they are. The size of the corneocytes we actually use to objectively measure the skin's renewal and desquamation (shedding of cells) process. These dead (keratin containing) skin cells may absorb water and swell. The lower layer with living cells doesn't swell up. As top layer (which is increased in size) is still attached to the layer beneath, a wrinkly pattern is formed. The layer of dead skin cells is thicker at the palms of our hands and soles of our feet, the wrinkling effect is more evident. This response occurs more quickly in freshwater than seawater. Moreover, when we are exposed to water for a longer time, the water-repelling film on top of the outer layer of the skin may get impaired. The sympathetic nervous system / microcirculation theory It's known that there is a relationship between the wrinkling-effect and blood vessels constricting (narrowing) below the skin. When hands and feet are soaked in water, the nerve fibres in the skin shrink and the body temperature regulators loses volume. Therewith the top layer of the skin is pulled downward and the wrinkling pattern is formed. It is proven that wrinkling-effect response is impaired, if the nerves and/or blood vessels are damaged. Therewith the wrinkling effect can even be used to determine proper functioning of the sympathetic nervous system and/or skin's microcirculation. There is evidence that the wrinkling effect is impaired in patients suffering from diabetes: link to article. Regardless the cause, aquatic wrinkles disappear fast and the skin returns to normal once the water has evaporated. Take care. 7/15/2018 Comments Facial oils bad for skin?!
Facial oils are a trending skin care product at the moment, loved and recommended by many "beauty guru's" and skin care experts. This is why I found it very interesting to read a comment written by a well respected dermatologist claiming that face oils would stifle skin renewal and exfoliation and would make skin dull over time. She must have a reason why she is saying this, and that's why I looked into this a little bit deeper.
To start with, I've done own research (not just me) with a facial oil, included many testers and found many benefits and no draw backs during the duration of the study. Moreover, I jumped out of my chair (literally) when I saw the visible results from the clinical photography, no joke! We've found that the oil (a combination of Argan oil and Lady's Thistle oil) improves moisture, elasticity and firmness, supports skin resilience, making the skin feel smooth and look more radiant. There was even a reduction of comedones detected. The results were published in a poster, accepted by the European Academy of Dermatology and Venereology in 2016. If you are an impatient person, and demand a fast answer, I can spill the tea right now: I've found no data to support that facial oils would stifle skin renewal and exfoliation, but the opposite. Moisturisers absolutely influence the skin barrier function and TEWL (transepidermal water loss - which is used to measure the skin barrier function). A good barrier function (confirmed by low TEWL), positively contributes to the skin cell renewal process, which includes skin exfoliation process. Very dry skin has an increased TEWL, and so does very well hydrated skin. There is simply more water on the skin surface to evaporate. A high TEWL with very well hydrated skin can therefore give the impression of an impaired barrier function and thus give a "false positive". This phenomenon is nicely explained in a publication by Marie Loden "Effect of moisturizers on epidermal barrier function". Looking at non fragrance plant oils also called fixed oils, there are many and they are all different, so it's impossible to generalise. Many plant oils, like almond, jojoba, soybean and avocado oils mostly remain on the skin surface. Even without penetrating deeper into the outer layer of the skin (called epidermis), the occlusive effect of plant oils will reduce water evaporation from the skin and help the growth of the cells of the top layer called keratinocytes. They actually support the skin barrier and therewith skin cell renewal. Part of the skin cell renewal is a process called desquamation, which is skin's natural exfoliation of dead skin cells. Helping this process will make skin appear more radiant and smooth, not duller. The benefits of plant oils are supported in many publications, one of which is found in the International Journal of Molecular Sciences anti inflammatory skin barrier repair effects by Tzu-Kai Lin 2017. Argan oil One of the most popular and well researched fixed oils is organ oil. It contains oleic and linoleic fatty acids. Both are part of our skin's natural intercellular lipid-enriched matrix or skin barrier. Linoleic acid (an omega 6 fatty acid) is in fact the most abundant polyunsaturated fatty acid. Our skin barrier is protecting our skin from water loss and penetration of external agressors. Thus the skin barrier keeps the good stuff in and bad stuff out. Linoleic acid plays a direct role in maintaining the integrity of this skin barrier. Some research shows that oleic acid may indeed disrupt the skin barrier and act as an permeability enhancer, helping other ingredients to penetrate deeper. When oleic acid is continuously applied, it could lead to barrier problems. Another ingredient in argan oil is tocopherol or vitamin E. Tocopherol is well known for it's antioxidative effect (neutralising damaging free radicals from pollution or sun which cause premature ageing) and lesser known for supporting the skin barrier. Daily topical application of argan oil (the finished product which contains multiple ingredients) has shown to improve skin elasticity (firmness), improve the skin hydration by restoring the barrier function and maintaining water-holding capacity. Furthermore it has a softening and relaxing effect on skin. Lady's Thistle oil The oil of the Milk Thistle plant (also known as Silybum Marianium) is a common ingredient in anti-aging skincare. It contains skin barrier supporting Linoleic acid and is known to nourish skin and improve radiance. Facial oils are certainly not for everybody, but in general skin will benefit from a cold pressed fixed plant oil or a mixture. Don't smother skin with oils, just apply a few drops by itself on the skin prior or after your moisturiser, or mix a few drops with your moisturiser of foundation. Hope you enjoy radiant skin & take care. 
Understandably we want to get rid of pimples as soon as possible and sometimes apply harsh products to our skin in order to shrink them.
Depending on which ingredient is used, you might inflict injury to the skin and it's barrier which is very comparable to a mild burn. There is even a phenomenon called "toothpaste burn". During the healing process there is a risk of scarring. A study published by Tan J. et al in JJD 2017 shows that up to >87% of patients with mild to moderate acne reports atrophic scarring (sunken scar) to some degree. A healthy skin barrier and well hydrated skin will support a the healing process. However, as the barrier is impaired and the skin dried out, the skin's regeneration and healing process will take longer. Therefore be careful with "shrinking" pimples. The same applies for "popping" pimples, as this method by definition will cause injury to the skin. Picking and squeezing pimples will further irritate the skin tissue and delay proper healing. The risk of scarring is increased when the tissue is inflamed. A recent study of prevalence and risk factors of acne scarring confirmed that there is a relationship between the time between onset and effective treatment. Acne scars can be more difficult to treat than acne! It's better to seek expert advice if you have problematic skin. Take care. |
CategoriesAll Acne Ageing Aquatic Wrinkles Armpits Biostimulators Blue Light & HEVIS Cleansing CoQ10 Cosmetic Intolerance Syndrome Deodorant Dermaplaning Diabetes Dry Skin Evidence Based Skin Care Exfoliation Exosomes Eyes Face Or Feet? Facial Oils Fibroblast Fingertip Units Gendered Ageism Glycation Gua Sha Hair Removal Healthy Skin Heat Shock Proteins Hormesis Humidity Hyaluron Hyaluronidase Hypo-allergenic Indulging Jade Roller Licochalcone A Luxury Skin Care Lymphatic Vessel Ageing Malar Oedema Menopause Mitochondrial Dysfunction Mood Boosting Skin Care Neurocosmetics Ox Inflammageing PH Balance Skin Photo Biomodulation Polynucleotides Psoriasis Regenerative Treatments Review Safety Scarring Sensitive Skin Skin Care Regimen Skin Flooding Skin Hydration Skin Senescence Skip-Care Sleep Slugging Sunscreen Tanning Under Eye Bags Vitamin C Well Ageing Skin Care Wound Healing Wrinkles
Archives
April 2024
|


 RSS Feed
RSS Feed
