
Mitochondria are the "powerhouses" or "lungs" of our cells and bioenergetic semi-autonomous organelles with their own genomes and genetic systems. [1] They are responsible for generating the energy that fuels a wide range of cellular processes in the skin, including cell signaling, pigmentation, wound healing, barrier integrity [2], metabolism and quality control. [3] Mitochondria exist in each cell of the body. Their primary role is cellular respiration; a process converting the energy in nutrients (like glucose) into a usable form of energy called ATP or Adenosine Triphosphate. Mitochondria are particularly abundant in the skin, reflecting the skin's high metabolic demand. When the functionality of mitochondria is impaired or declines, it impacts skin's vitality, health and beauty. Mitochondrial dysfunction is 1 of the 12 hallmarks of skin ageing.
The skin is particularly susceptible to mitochondrial stress due to its constant exposure to environmental insults, such as UV radiation, pollution, and other oxidative stressors. These factors can damage mitochondrial DNA, leading to increased production of reactive oxygen species (ROS) and disrupting the delicate balance of cellular processes. [4] In aged post-mitotic cells, heavily lipofuscin-loaded lysosomes perform poorly, resulting in the enhanced accumulation of defective mitochondria, which in turn produce more reactive oxygen species causing additional damage (the mitochondrial-lysosomal axis theory). [5] Optimal mitochondrial function is indispensable for sustaining the specialized functions of each cell type, like keratinocyte differentiation, fibroblast ECM production, melanocytes melanin production and distribution, immune cell surveillance, sebocytes and adipocytes. [6] Mitochondrial dysfunction is both directly and indirectly linked to chronological ageing and photo-ageing. [7] As mitochondrial function declines, the skin's ability to regenerate and repair itself is decreased. [2=1] This results in visible signs of aging, such as wrinkles, loss of elasticity, dryness, uneven pigmentation, melasma, age spots, lipomas, impaired wound healing. [2-4-5-8-9] Mitochondrial dysfunction also has been implicated in skin conditions like acne, eczema, lupus, psoriasis, vitiligo, atopic dermatitis and even skin cancer. [10] Ageing is associated with changes in mitochondrial morphology, including [6]
Good mitochondrial function or metabolism: [7]
Dysfunctional Mitochondria: [7]
Mitochondrial proteins Mitochondria contain >1,100 different proteins (MitoCoP) that often assemble into complexes and supercomplexes such as respiratory complexes and preprotein translocases. The chaperones Heat Shock Proteins HSP60-HSP10 are the most abundant mitochondrial proteins. [3] Small heat shock proteins form a chaperone system that operates in the mitochondrial intermembrane space. Depletion of small heat shock proteins leads to mitochondrial swelling and reduced respiration. [14] Mitochondrial hyperpigmentation Emerging research has shed light on the intricate relationship between mitochondrial dysfunction and the development of hyperpigmentation, a condition characterized by the overproduction and uneven distribution of melanin in the skin. One of the key mechanisms underlying this connection is the role of mitochondria in the regulation of melanogenesis, the process by which melanin is synthesized. Mitochondria are involved in the production of various cofactors and signaling molecules that are essential for the activity of tyrosinase, the rate-limiting enzyme in melanin synthesis. [15] When mitochondrial function is impaired, it can lead to an imbalance in the production and distribution of these cofactors and signaling molecules, ultimately resulting in the overproduction and uneven deposition of melanin in the skin. [15] This can manifest itself as age spots, melasma, and other forms of hyperpigmentation. The link between mitochondrial dysfunction and hyperpigmentation has been further supported by studies on genetic disorders that involve mitochondrial dysfunction, such as mitochondrial DNA depletion syndrome. In these conditions, patients often exhibit a range of pigmentary skin changes, including patchy hyper- and hypopigmentation, as well as reticular pigmentation. [16] Mitochondrial crosstalk and exosomes Mitochondria can crosstalk and move beyond cell boundaries. [17] Mitochondria-derived material might be transferred to neighboring cells in the form of cell-free mitochondria or included in extracellular vesicles [18-19]. This process supports cellular repair and contributes to vital mitochondrial functions. Besides restoring stressed cells and damaged tissues due to mitochondrial dysfunction, intercellular mitochondrial transfer also occurs under physiological and pathological conditions. [20] The transfer of active mitochondria from mesenchymal stem cells (MSCs) has been identified as a repair mechanism for rejuvenating damaged skin fibroblasts. [21] MITOCHONDRIAL SUPPORT Q10 or Coenzyme Q10 (CoQ10) Q10 is part of the mitochondrial respiration chain and essential for cellular energy production. About 95% of our cellular energy is generated with support of Q10, which is produced by the human body itself. During skin ageing, both the cellular energy production and levels of Q10 are declined. Q10 is a powerful anti-oxidant [22], thus protecting cells from oxidative stress and damage and has proven to be able to "rescue" senescent cells by decreasing elevated senescent markers like p21 levels and β-Galactosidases positive cell numbers (in-vitro). Q10 is bio-active, increasing collagen type I and elastin production. [23=8] Q10 can be supplemented via nutrition, however also via topical application and is considered an evidence based active ingredient in skin care products. Ubiquinol (reduced form) shows higher bioavailability compared to ubiquinone (oxidized form). [23] Glutathione Glutathione is formed in cell's cytoplasm from glutamic acid, cysteine and glycine. It is present in 2 forms: reduced (GSH) and oxidized (GSSG). Reduced GSH is an active anti-oxidant, while the presence of inactive GSSG is increased under oxidative stress. The ratio between GSH and GSSH is considered a measure of oxidative stress. Glutathione participates in redox reactions, acts as co-factor of many anti-oxidant enzymes and is the most important non-enzymatic anti-oxidant, essential for synthesis of proteins and DNA. Low Glutathione results in accelerated ageing and inflammatory skin diseases. Mitochondrial glutathione (mGSH) is the main line of defense for the maintenance of the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunction and cell death. [24] Glutathione can be increased via supplementation via precursors cysteine or N-acetylcysteine (not recommended for pregnant women) or the reduced form of Glutathione itself, or increased via topical active ingredients like Licochalcone A. [25] Nicotinamide NR nicotinamide ribosome which is the precursor of NMN nicotinamide mononucleotide which is the precursor of NAD+ nicotinamide adenine dinucleotide all could have a protective effect on mitochondria. Nicotinamide adenine dinucleotide is present in living organisms as ions NAD+ and NADP+ and in reduced forms NADH and NADPH. NADH is a cofactor of processes inside mitochondria:
Resveratrol Although systemically Resveratrol promotes mitochondrial biogenesis. [27] Other data shows that UVA (14 J/cm(2)) along with resveratrol causes massive oxidative stress in mitochondria. As a consequence of oxidative stress, the mitochondrial membrane potential decreases which results in opening of the mitochondrial pores ultimately leading to apoptosis in human keratinocytes. [28] Red light therapy By incorporating red light therapy into your skin care routine, you can help to counteract the damaging effects of mitochondrial dysfunction and support the skin's natural renewal processes. Next to the use of sunscreens (especially when containing Licochalcone A), CoQ10, anti-oxidants and Nicotinamide, emerging treatments like mitochondrial transfer and therapies focused on improving mitochondrial quality control processes are being investigated as potential solutions for preventing and addressing mitochondrial dysfunction in the skin. As we continue to explore the 12 hallmarks of ageing skin, I am confident that we will gain valuable insights and develop breakthrough innovations that will improve skin quality, health, beauty and vitality. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References
Comments

Glycation is one of the basic root causes of endogeneous (intrinsic) skin ageing and a very challenging one or almost impossible one to reverse. Glycation is an ageing reaction which begins in early life, developing clinical symptoms at around 30, and progressively accumulates in tissues and skin due to the glycated collagens that are difficult to be decomposed. Glycation occurs naturally in the body when sugars react with proteins and lipids to form advanced glycation end products (AGEs). AGEs can be exogenously ingested (through food consumption), inhaled via tobacco or endogenously produced and formed both intracellularly and extracellularly. AGE modifications lead to dermal stiffening, diminished contractile capacity of dermal fibroblasts, lack of elasticity in the connective tissues, contribute to hyperpigmentation and a yellowish skin appearance. The formation of AGEs is amplified through exogenous factors, e.g., ultraviolet radiation.
AGEs cause changes in the skin through 3 processes:
One study published in the Journal of Investigative Dermatology found that levels of AGEs were higher in the skin of older individuals compared to younger ones. The study also showed that there was a correlation between the level of AGEs and the severity of skin ageing. This suggests that inhibiting the production or accumulation of AGEs in the skin is a potential target for anti-ageing interventions or skin ageing management. AGEs are complex and heterogeneous, more than a dozen AGEs have been detected (however not all) in tissues and can be divided into three categories according to their biochemical properties. AGEs are formed through four pathways:
GLYCATION INHIBITION IS KEY AGEs can be crosslinked through side chains to form a substance of very high molecular weight, which is not easily degraded. The consequences from skin glycation are irreversible. This makes prevention or inhibition of the process the best potential strategy to maintain skin health and ageing skin management. One way to do this is by altering the diet to reduce the intake of sugars and carbohydrates, which are known to contribute to glycation. Several studies have found that reducing sugar intake can result in significant improvements in skin health, including reducing wrinkles and improving skin texture. 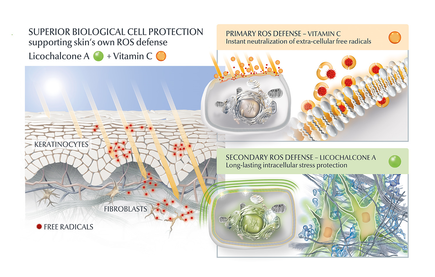
AGE inhibitors
Another potential strategy is the use of topical agents that inhibit the formation or accumulation of AGEs in the skin. One study published in the Journal of Cosmetic Science found that a cream containing carnosine, a peptide that inhibits glycation, improved skin elasticity and reduced the appearance of wrinkles in individuals with ageing skin. Skincare containing NAHP or Acetyl Hydroxyproline inhibits the formation of AGEs significantly (in vitro), most likely through a mechanism where NAHP competes with the proteins for the sugar. Finally, NAHP sacrifices itself in place of the proteins and gets (at least partially) glycated. NAHP also prevents loss of cellular contractile forces in a glycated in vitro dermis model and counteracts the diminished cell-matrix interaction that is caused by glyoxal-induced AGE formation. Anti-Oxidants Moreover, I would suggest to combine those ingredients with an ingredient like Licochalcone A. Numerous high ranked publications support that Licochalcone A protects cells from oxidative stress mediated by e.g. UV and HEVIS (blue light) induced reactive oxidative species (ROS). Due to the activation and nuclear translocation of the transcription factor NrF2, the expression of anti-inflammatory, antioxidant and detoxifying enzymes are induced. These enzymes protect the skin cells (like keratinocytes and fibroblasts) from ROS-induced damage, like lipid peroxidation and DNA as well as protein damage. If Licochalcone A is combined with L-Ascorbic Acid, (the most active form of Vitamin C), it supporting skin's own collagen production, provides superior biological cell protection amongst other relevant benefits. My absolute favourite product is Eucerin Hyaluron-Filler Vitamin C Booster which I use daily as a serum in my morning routine. GLYCATION AND SKIN HEALTH Acne In addition to its role in ageing, glycation in the skin has also been linked to a range of skin health problems. One study published in the Journal of Cosmetic Dermatology found that the level of AGEs in the skin was significantly higher in individuals with acne than in those without acne. The study also showed that treating acne with a topical antibiotic significantly reduced the levels of AGEs in the skin. Atopic Dermatitis Another study published in the Journal of Investigative Dermatology found that individuals with atopic dermatitis had higher levels of AGEs in their skin than healthy individuals. This suggests that glycation may play a role in the development of inflammatory skin conditions. Diabetes + Woundhealing The correlation between high sugar levels and skin ageing can be seen in diabetic patients, where one-third of this population has skin complications. A prominent feature of ageing human skin is the fragmentation of collagen fibers, which severely damages the structural integrity and mechanical properties of the skin. Elevated levels of MMP-1 and MMP-2 and higher crosslinked collagen in the dermis of diabetic skin lead to the accumulation of fragmented and crosslinked collagen, thereby impairing the structural integrity and mechanical properties of dermal collagen in diabetes. Collagen crosslinking makes it impossible for them to easily repair, resulting in reduced skin elasticity and wrinkles. Keratinocytes and fibroblasts are the main cells involved in wound healing, but due to the high glucose (HG) microenvironment in diabetics, the functional state of these cells is impaired, thereby accelerating cellular senescence (programmed cell death). Conclusion We can't completely stop the glycation process, therefore it's important that we inhibit it from a young age onwards, hence monitor the sugar intake of our children, use daily SPF and invest in good dermo-cosmetic products containing ingredients like NAHP and powerful anti-oxidants like L-Ascorbid Acid (Vitamin C is needed for the production of collagen) and Licochalcone A (also anti-inflammatory). Preventing signs of ageing, specifically caused by glycation is most effective. If your skin shows (advanced) signs of ageing, you can get visible improvement using skin component (hyaluron, collagen and elastin) bio-stimulating ingredients like Retinol, Bakuchiol, Arctiin, Creatine or Glycine Saponin. Consult your dermatologist if you wish to improve your skin's appearance or skin health issues. Take care Special thanks: Ph.D. dr Julia M. Weise Manager Biological Testing & Dorothea Schweiger Lab Manager Facial Skin Biology Beiersdorf HQ Hamburg 
Psoriasis occurs in many different forms and levels of severity. The first signs may appear between the age of 15 and 35 and 75% of patients are diagnosed before the age of 46 according to the World Health Organisation. As there is no cure for the disease, the highest prevalence is seen in a more mature age group age 50-69. Many of the treatments which are part of the standard treatment guidelines for psoriasis cause as a side effect premature ageing skin. For example PUVA, next to being an effective treatment, does cause (severe) photo-damage. Many patients will undergo such treatments on and off or continuously throughout their life.
Although the primary goal of dermatology is to improve the functional attributes of the skin (health) and lessen the tremendous burden psoriasis may cause, ultimately one aims to improve the skin's physical attributes (appearance). Ageing skin is a biological degenerative process which influences the activity of collagen and hyaluronic acid producing cells (mainly fibroblasts) and leads to a decrease of skin components like collagen, hyaluron and functional elastin. Effective anti-aging skincare can support the protection of those cells and skin components (anti-oxidants and SPF) and thus slow down the fastened degenerative process. Some active ingredients (for example biologically active Glycine Saponin and Arctiin) have proven to effectively stimulate fibroblasts in the production of collagen and hyaluronic acid and thus replenish dermal components. Loss of those components and photo-damage eventually lead to visible signs of ageing. Although ageing skin is natural, premature ageing skin isn't necessary. Most patients will probably already use a moisturising facial skincare product. It makes sense to recommend anti-ageing skincare instead to be used in conjunction with treatments which as a side effect cause premature ageing skin, particularly for exposed areas like the face. As psoriasis oftentimes doesn't occur in the face (except in the hairline), anti-ageing skincare will pose a low to no risk to aggravate facial skin and there are anti-ageing skincare products available which have proven to be suitable for psoriasis patients. Take care. |
CategoriesAll Acne Ageing Aquatic Wrinkles Armpits Biostimulators Blue Light & HEVIS Cleansing CoQ10 Cosmetic Intolerance Syndrome Deodorant Dermaplaning Diabetes Dry Skin Evidence Based Skin Care Exfoliation Exosomes Eyes Face Or Feet? Facial Oils Fibroblast Fingertip Units Gendered Ageism Glycation Gua Sha Hair Removal Healthy Skin Heat Shock Proteins Hormesis Humidity Hyaluron Hyaluronidase Hypo-allergenic Indulging Jade Roller Licochalcone A Luxury Skin Care Lymphatic Vessel Ageing Malar Oedema Menopause Mitochondrial Dysfunction Mood Boosting Skin Care Neurocosmetics Ox Inflammageing PH Balance Skin Photo Biomodulation Polynucleotides Psoriasis Regenerative Treatments Review Safety Scarring Sensitive Skin Skin Care Regimen Skin Flooding Skin Hydration Skin Senescence Skip-Care Sleep Slugging Sunscreen Tanning Under Eye Bags Vitamin C Well Ageing Skin Care Wound Healing Wrinkles
Archives
April 2024
|


 RSS Feed
RSS Feed
