
Blue light, is also known as high-energy visible (HEV) light and is the most energetic part of the visible light spectrum (380 - 700 nm) with wavelengths ranging from indigo or ultramarine light 420-440 nanometers, blue light 450-495 nanometers to cyan light 480 - 520 nanometers. Blue light has lower energy than ultraviolet (UV) radiation (280–400 nm) and can reach further into the dermis, up to the depth of 1 mm. [1] Sunlight is the primary natural source of blue light. Up to 50% of the damaging oxidative stress in human skin is generated in the VIS spectrum and the other 50% by UV light [2], contributing to premature ageing, ox-inflammageing and hyperpigmentation like age spots.
Blue light from electronic devices The use of electronic devices has led to increased exposure to artificial blue light sources, however the amount of blue light emitted during the conventional use of electronic devices is by far not enough to trigger harmful skin effects. If you sit in front of a monitor uninterrupted for a week at a distance from the screen of approximately 30 cm, this would be the same as the blue light intensity of spending one minute outside on a sunny day in Hamburg Germany at around midday at midsummer. If you hold a smartphone right next to the skin, the intensity does increase, but it would still take approximately 10 hours of uninterrupted use to match the effect on the skin of just one minute of sunlight. The emissions from electronic devices are barely noticeable in comparison to natural blue light directly from the sun and are, thus negligible. However, blue light or HEV light from sunlight can be harmful for skin. Dr Ludger Kolbe Chief Scientist for Photobiology and his team at Beiersdorf AG did pioneering research regarding the harmful effects of HEVIS. [3-4] I would also like to take the opportunity to debunk an important myth at the start of this article as infrared or near infrared light does not induce damaging free radicals (even in high amounts), there is no such thing "infra-ageing" as a result or IR and in fact red light photobiomodulation supports skin rejuvenation. Read more Direct effects of blue light and HEV Light on skin Blue light and HEV light can have both beneficial and detrimental effects on the skin. The most significant direct effects are mediated through their interaction with chromophores, such as flavins, porphyrins, and opsins, which can trigger the overproduction of reactive oxygen species (ROS), reactive nitrogen species (RNS). and hyperpigmentation. Reactive oxygen and nitrogen species cause DNA damage and modulate the immune response. [1] This oxidative stress can lead to: Photo-ageing: Exposure to blue light and HEV light can induce premature skin aging, causing wrinkles, fine lines, and loss of elasticity. Hyperpigmentation: Blue light and HEV light can stimulate melanin production, leading to uneven skin tone and the development of age spots or other forms of hyperpigmentation. DNA Damage: The ROS and RNS generated by blue light and HEV light can cause DNA damage, plus potentially increase the risk of skin cancer. Inflammation: The oxidative stress triggered by blue light and HEV light can cause an inflammatory response in the skin, exacerbating conditions like acne, eczema, and psoriasis. Molecular and physiological mechanisms of direct blue light effects on the skin [1]
Indirect effects of blue light and HEV Light on skin Blue light and HEV light can also have indirect effects on the skin by disrupting the body's circadian rhythms. This occurs via both the central mechanism, which involves stimulation of light-sensing receptors located in the retina, and via the peripheral mechanism, which involves direct interaction with skin cells. By disrupting the normal circadian rhythm, blue light can negatively affect the skin's natural overnight repair and regeneration processes. [1] The circadian rhythm has been shown to affect multiple cellular and physiological processes occurring in the skin:
Molecular mechanisms of indirect effects of blue light on the skin [1]
Ideal daytime & nighttime skin care regimen When considering cosmetic interventions, a strategy of daytime protection plus defense and night-time repair may be optimal. The skin's own repair mechanisms, such as base excision repair and nucleotide excision repair, attempt to mitigate blue light induced DNA damage. [12] Daytime protection plus defense Of course prevention and/or reduction of blue light exposure from sunlight is key. Reduce the time spent on electronic devices, especially before bedtime, can help minimize the disruption of circadian rhythms and the indirect effects of blue light and HEV light on the skin. Against premature ageing and hyperpigmentation an evidence based effective approach could be the daily use of tinted broadband sunscreen preferably containing Licochalcone A (the most effective anti-oxidant reducing damaging free radical activity from both UV and blue light and moreover protects against collagenase MMP-1 expression) strengthening skin's biological defense [4-5-6-7], while iron oxides in colour pigments provide physical protection against blue light (like zinc oxide and titanium dioxide). Against hyperpigmentation there are (tinted) sunscreens which on top contain the most potent human tyrosinase inhibitor found in dermatological skin care called Thiamidol® [8-9] and one of the 3 ingredients in the "new Kligman Trio" (NT) [18] and Glycyrrhetinic Acid which supports skin's DNA repair and skin pigmentation [10] and inhibits hyaluronidase activity (HYAL1). Most regular sun filters used in sunscreen don't offer any protection against blue light, however according to the website of BASF the chemical UV filters Tinosorb® A2B and Tinosorb® M can reduce the exposure to blue light. [11] Scattering and absorption of blue light [5] The penetration depth of visible light is influenced by the reflection, scattering, and absorption mediated not only by the skin’s physical barrier but also by the VL chromophores in the skin and Fitzpatrick skin or photo-type (FST). The primary VL-scatter and absorption molecules in the skin include hemoglobin, melanin, bilirubin, carotene, lipids, and other structures, including cell nuclei and filamentous proteins like keratin and collagen. Melanin and keratins are the primary VL absorbers and scatterers in the epidermis, while hemoglobin is the dominant absorber, and collagen is the major VL scatter in the dermis. Melanin's absorption spectrum ranges from 200 to 900 nm, with the peak absorption varying based on melanin moiety.. This means that individuals with darker skin types, which have higher melanin content, are more prone to hyperpigmentation from blue light or VIS due to the greater absorption and scattering of VIS in their skin on top of the previously mentioned higher levels of tyrosinase–DCT complexes leading to increased melanogenesis, leading to both transient and long-lasting pigmentation [13], dependent upon the total dose and exacerbation of melasma especially in individuals with FSTs III to VI. Blue light tanning Recent data demonstrate synergistic effects between VL and UV-A on erythema and pigmentation. VL-induced pigmentation is more potent and more sustained than UVA1-induced pigmentation in darker skin tones.Typically, three mechanisms are involved in the responsive reaction of melanocytes to VL, with increased melanin content: immediate pigment darkening (IPD), persistent pigment darkening (PPD), and delayed tanning (DT). [15] Read more. VL can also exacerbate post inflammatory hyperpigmentation (study with FST IV and V). [16] Blue light therapy While the detrimental effects of blue light and HEV light on the skin have been well-documented, these wavelengths have also shown promise in the treatment of certain skin conditions. In controlled clinical settings, blue light has been used to: Treat Acne: Blue light can reduce the growth of Propionibacterium acnes, the bacteria responsible for acne, and has an anti-inflammatory effect. Manage Psoriasis and Atopic Dermatitis: Blue light has been found to have an anti-inflammatory and antiproliferative effect, making it potentially beneficial for the treatment of these chronic inflammatory skin diseases. Reduce Itch: Some studies have suggested that blue light may help alleviate the severity of itching in certain skin conditions. The optimal protocols for blue light therapy are still being developed, and the long-term safety of this treatment modality requires further investigation and should not be initiated without HCP recommendation and monitoring. Vitiligo: Blue light therapy via LEDs can stimulate repigmentation in patients with vitiligo with minimal adverse events, however larger studies are needed. [17] Overall, the research suggests that prolonged or excessive exposure to high-energy blue light, can have negative long-term effects on skin structure, function, and appearance in all phototypes. As our understanding of the individual variations in skin's response to blue light exposure deepens, the development of personalised or tailored effective solutions become increasingly more tangible. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References
Comments

Mitochondria are the "powerhouses" or "lungs" of our cells and bioenergetic semi-autonomous organelles with their own genomes and genetic systems. [1] They are responsible for generating the energy that fuels a wide range of cellular processes in the skin, including cell signaling, pigmentation, wound healing, barrier integrity [2], metabolism and quality control. [3] Mitochondria exist in each cell of the body. Their primary role is cellular respiration; a process converting the energy in nutrients (like glucose) into a usable form of energy called ATP or Adenosine Triphosphate. Mitochondria are particularly abundant in the skin, reflecting the skin's high metabolic demand. When the functionality of mitochondria is impaired or declines, it impacts skin's vitality, health and beauty. Mitochondrial dysfunction is 1 of the 12 hallmarks of skin ageing.
The skin is particularly susceptible to mitochondrial stress due to its constant exposure to environmental insults, such as UV radiation, pollution, and other oxidative stressors. These factors can damage mitochondrial DNA, leading to increased production of reactive oxygen species (ROS) and disrupting the delicate balance of cellular processes. [4] In aged post-mitotic cells, heavily lipofuscin-loaded lysosomes perform poorly, resulting in the enhanced accumulation of defective mitochondria, which in turn produce more reactive oxygen species causing additional damage (the mitochondrial-lysosomal axis theory). [5] Optimal mitochondrial function is indispensable for sustaining the specialized functions of each cell type, like keratinocyte differentiation, fibroblast ECM production, melanocytes melanin production and distribution, immune cell surveillance, sebocytes and adipocytes. [6] Mitochondrial dysfunction is both directly and indirectly linked to chronological ageing and photo-ageing. [7] As mitochondrial function declines, the skin's ability to regenerate and repair itself is decreased. [2=1] This results in visible signs of aging, such as wrinkles, loss of elasticity, dryness, uneven pigmentation, melasma, age spots, lipomas, impaired wound healing. [2-4-5-8-9] Mitochondrial dysfunction also has been implicated in skin conditions like acne, eczema, lupus, psoriasis, vitiligo, atopic dermatitis and even skin cancer. [10] Ageing is associated with changes in mitochondrial morphology, including [6]
Good mitochondrial function or metabolism: [7]
Dysfunctional Mitochondria: [7]
Mitochondrial proteins Mitochondria contain >1,100 different proteins (MitoCoP) that often assemble into complexes and supercomplexes such as respiratory complexes and preprotein translocases. The chaperones Heat Shock Proteins HSP60-HSP10 are the most abundant mitochondrial proteins. [3] Small heat shock proteins form a chaperone system that operates in the mitochondrial intermembrane space. Depletion of small heat shock proteins leads to mitochondrial swelling and reduced respiration. [14] Mitochondrial hyperpigmentation Emerging research has shed light on the intricate relationship between mitochondrial dysfunction and the development of hyperpigmentation, a condition characterized by the overproduction and uneven distribution of melanin in the skin. One of the key mechanisms underlying this connection is the role of mitochondria in the regulation of melanogenesis, the process by which melanin is synthesized. Mitochondria are involved in the production of various cofactors and signaling molecules that are essential for the activity of tyrosinase, the rate-limiting enzyme in melanin synthesis. [15] When mitochondrial function is impaired, it can lead to an imbalance in the production and distribution of these cofactors and signaling molecules, ultimately resulting in the overproduction and uneven deposition of melanin in the skin. [15] This can manifest itself as age spots, melasma, and other forms of hyperpigmentation. The link between mitochondrial dysfunction and hyperpigmentation has been further supported by studies on genetic disorders that involve mitochondrial dysfunction, such as mitochondrial DNA depletion syndrome. In these conditions, patients often exhibit a range of pigmentary skin changes, including patchy hyper- and hypopigmentation, as well as reticular pigmentation. [16] Mitochondrial crosstalk and exosomes Mitochondria can crosstalk and move beyond cell boundaries. [17] Mitochondria-derived material might be transferred to neighboring cells in the form of cell-free mitochondria or included in extracellular vesicles [18-19]. This process supports cellular repair and contributes to vital mitochondrial functions. Besides restoring stressed cells and damaged tissues due to mitochondrial dysfunction, intercellular mitochondrial transfer also occurs under physiological and pathological conditions. [20] The transfer of active mitochondria from mesenchymal stem cells (MSCs) has been identified as a repair mechanism for rejuvenating damaged skin fibroblasts. [21] MITOCHONDRIAL SUPPORT Q10 or Coenzyme Q10 (CoQ10) Q10 is part of the mitochondrial respiration chain and essential for cellular energy production. About 95% of our cellular energy is generated with support of Q10, which is produced by the human body itself. During skin ageing, both the cellular energy production and levels of Q10 are declined. Q10 is a powerful anti-oxidant [22], thus protecting cells from oxidative stress and damage and has proven to be able to "rescue" senescent cells by decreasing elevated senescent markers like p21 levels and β-Galactosidases positive cell numbers (in-vitro). Q10 is bio-active, increasing collagen type I and elastin production. [23=8] Q10 can be supplemented via nutrition, however also via topical application and is considered an evidence based active ingredient in skin care products. Ubiquinol (reduced form) shows higher bioavailability compared to ubiquinone (oxidized form). [23] Glutathione Glutathione is formed in cell's cytoplasm from glutamic acid, cysteine and glycine. It is present in 2 forms: reduced (GSH) and oxidized (GSSG). Reduced GSH is an active anti-oxidant, while the presence of inactive GSSG is increased under oxidative stress. The ratio between GSH and GSSH is considered a measure of oxidative stress. Glutathione participates in redox reactions, acts as co-factor of many anti-oxidant enzymes and is the most important non-enzymatic anti-oxidant, essential for synthesis of proteins and DNA. Low Glutathione results in accelerated ageing and inflammatory skin diseases. Mitochondrial glutathione (mGSH) is the main line of defense for the maintenance of the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunction and cell death. [24] Glutathione can be increased via supplementation via precursors cysteine or N-acetylcysteine (not recommended for pregnant women) or the reduced form of Glutathione itself, or increased via topical active ingredients like Licochalcone A. [25] Nicotinamide NR nicotinamide ribosome which is the precursor of NMN nicotinamide mononucleotide which is the precursor of NAD+ nicotinamide adenine dinucleotide all could have a protective effect on mitochondria. Nicotinamide adenine dinucleotide is present in living organisms as ions NAD+ and NADP+ and in reduced forms NADH and NADPH. NADH is a cofactor of processes inside mitochondria:
Resveratrol Although systemically Resveratrol promotes mitochondrial biogenesis. [27] Other data shows that UVA (14 J/cm(2)) along with resveratrol causes massive oxidative stress in mitochondria. As a consequence of oxidative stress, the mitochondrial membrane potential decreases which results in opening of the mitochondrial pores ultimately leading to apoptosis in human keratinocytes. [28] Red light therapy By incorporating red light therapy into your skin care routine, you can help to counteract the damaging effects of mitochondrial dysfunction and support the skin's natural renewal processes. Next to the use of sunscreens (especially when containing Licochalcone A), CoQ10, anti-oxidants and Nicotinamide, emerging treatments like mitochondrial transfer and therapies focused on improving mitochondrial quality control processes are being investigated as potential solutions for preventing and addressing mitochondrial dysfunction in the skin. As we continue to explore the 12 hallmarks of ageing skin, I am confident that we will gain valuable insights and develop breakthrough innovations that will improve skin quality, health, beauty and vitality. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Many people associate a tan with health, beauty and an active lifestyle. Although a moderate dose of solar radiation is indispensable for our health, unfortunately, there is no such thing as a real "healthy tan" or "healthy sun-kissed glow" as it is always a visible sign of skin damage. Tanning is a response by the skin to exposure to ultraviolet (UV) radiation (and HEV or Blue Light), either from natural sunlight or artificial sources like tanning beds which leads to photo-ageing, pigmentary disorders (like age spots or hyperpigmentation) and immunosuppression, hence skin cancer. When skin is exposed to sunlight: UV rays and high energy visible light (HEV) or also called Blue Light (the most energetic region of HEV), it produces more melanin, a pigment that darkens the skin as a (partial) protective mechanism to prevent further damage. The amount of artificial blue light emitted during the conventional use of electronic devices is not enough to trigger harmful skin effects. (Click here to read more)
MELANIN Melanin is only produced by cells called melanocytes, mostly distributed in the epidermal-dermal junction. Melanocytes contain specialized organelles called melanosomes to store and produce melanin. Melanosomes are transferred from the melanocytes to the neighboring keratinocytes, which are the most abundant cells in the epidermis. One melanin-forming melanocyte surrounded by 36 keratinocytes and a Langerhans cell is called the melano-epidermal unit. [1.2] Melanocytes use the amino acid tyrosine to produce melanin and protect epidermal keratinocytes and dermal fibroblasts from the damaging effects of solar radiation.. [13] The are two melanin pigment classes:
Differences in skin pigmentation do not result from differences in the number of melanocytes in the skin, as one might assume, but from differences in the melanogenic activity (melano-competence), the type of melanin produced in melanosomes (the ratio between eumelanin and pheomelanin differs per Fitzpatrick phototype) and the size, number and packaging of melanosomes, with melanin content of melanosomes ranging from 17.9% to 72.3%. [7] The amount of melanin is never enough for adequate photoprotection, and a "base tan" does not prevent sunburn. Particularly darker phototypes are more sensitive for the damaging effects of Blue Light. Both eumelanin and pheomelanin production are promoted by UV radiation and Blue Light and therefore sunscreens offering a combination of both UV (A + B) protection and Blue Light defense are recommended for all phototypes. TANNING PROCESS The skin's tanning process occurs in four distinct phases: [3]
ROLE OF UVA, UVB AND BLUE LIGHT One of the most important acute effects of UVR is DNA photodamage. UVA and UVB show different properties regarding their biological effects on the skin. [7] Shorter wavelengths (nm) correspond to higher energy. Infrared does not induce oxidative stress. Read more UVA radiation (320-400 nm) penetrates deeper into the skin and can induce indirect DNA damage by the generation of reactive oxygen species (ROS), leading to premature skin aging. UVA, in contrast to UVB is not filtered by window glass, is able to penetrate deeper into the skin and reach the dermis. They are present constantly, with relatively equal intensity, during all daylight hours throughout the year. It has been estimated that 50% of exposure to UVA occurs in the shade. UVA rays are less intense than UVB, but there are 30 to 50 times more of them. To produce the same erythemal response, approximately 1000 times more UVA dose is needed compared with UVB. [7] The bulbs used in tanning beds emit mostly UVA. UVB radiation (280-320 nm) is less prevalent than UVA, primarily affects the outermost layers of the skin, causing direct DNA damage (more potent than UVA) and triggers inflammatory responses that lead to increased melanin production. UVB radiation fluctuates throughout the day, are at their strongest at noon. and are more cytotoxic and mutagenic than UVA. The action spectrum for UV-induced tanning and erythema are almost identical, but UVA is more efficient in inducing tanning whereas UVB is more efficient in inducing erythema (redness). Dark skin is twice as effective compared to light skin in inhibiting UVB radiation penetration. [7] UVB helps the skin to produce Vitamin D. Blue light (400-500 nm) visible light accounts for 50% of sunlight [11] and can contribute to immediate, delayed, continuous and long-lasting pigmentation by activating melanocyte-specific photoreceptors and increasing melanin synthesis, particularly in individuals with darker (melano-competent) skin types [9], cause DNA damage [10] and generate damaging reactive oxygen species in both the epidermis and the dermis. [12] The effects may last longer than those induced by UVA and UVB radiation. Blue Light can penetrate even deeper than UVA and reach the hypodermis. Blue light therapy is used to target acne causing bacteria and inflammation, however the risks might outweigh the benefits especially in darker phototypes and it might worsen acne marks. EPIDERMIS AND DERMIS Both dermal fibroblasts and epidermal keratinocytes play a crucial role in regulating skin pigmentation and tanning response. [13 15] In comparison to epidermal tanning, dermal tanning is less visible, however more immediate. Dermal fibroblasts secrete various paracrine factors that regulate melanocyte function, survival, and melanin production. Factors like hepatocyte growth factor (HGF), nerve growth factor (NGF), stem cell factor (SCF), and basic fibroblast growth factor (bFGF) stimulate melanogenesis and pigmentation [14 15] Fibroblast senescence and altered secretory profiles in conditions like melasma contribute to abnormal pigmentation by stimulating melanogenesis. [15] Epidermal keratinocytes produce factors like α-melanocyte stimulating hormone (α-MSH) and Wnt1 that activate melanogenic pathways in melanocytes, leading to increased melanin synthesis and transfer to keratinocytes. [15 16]. Keratinocyte-derived exosomes can enhance melanin production by melanocytes. [16] Differences in autophagic activity between various keratinocytes also influences pigmentation. [15] Enjoy the sun, however protect your (and your children's) skin from a photo-damaging tan to remain skin health and beauty. Sunless self-tanning products containing dihydroxyacetone (DHA) or Erythrulose provide a safe alternative to achieve a "sun-kissed" glow. You can use after-sun skin care which helps to rehydrate, reduce damage of "sun-stressed" skin and support it's repair. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

In skin biology, senescence is a process by which a cell ages and permanently stops dividing but does not die. This is why they are also referred to as "zombie cells". Age-related accumulation of senescent cells is caused by of increased levels of senescence-inducing stressors and/or reduced elimination of senescent cells. Under normal physiological conditions, senescent cells play an important role maintaining cellular homeostasis and inhibiting proliferation of abnormal cells. However, over time, large numbers of zombie cells can build up in the skin and contribute to the overall reduction in skin's regenerative properties, impacting both its beauty and health.
There are 2 forms of cell senescence: Acute senescence: Senescent cells are produced in response to acute stressors to facilitate for example tissue repair, wound healing. They are cleared by our immune system. Chronic senescence: A not programmed process as response to prolonged stress or damage and these senescent cells are not cleared by our immune system, leading to the accumulation of zombie cells impacting our skin health and beauty. It has been suggested that inflammageing is mainly related to senescent cells and their associated SASP (Senescence-Associated Secretory Phenotype) which increase in the body with age and contribute to inflammageing. Senescent cells cause inflammageing and inflammageing causes cell senescence. [1] Senescence can be triggered by a number of stress signals to the cell [1]:
Mechanisms of skin cell senescence:
The presence of senescent cells accelerates the ageing process due to their communication with nearby cells through various molecules: [18]
Fibroblast senescence could be the main driver of the skin ageing. [3] The increased number of senescent fibroblasts results in the production of SASPs rich in pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-8, IL-18, matrix metalloproteinases (MMPs), and a variety of other inflammatory chemokines [2] resulting in the breakdown of collagen, loss of elasticity and wrinkle formation. [3] Autophagy in dermal fibroblasts is essential for maintaining skin balance and managing the ageing process, particularly in response to external stressors like UV radiation and particulate matter (PM), by repairing cellular machineries. [4] Insufficient autophagy leads to an exaggerated skin inflammation triggered by inflammasome activation, resulting in accelerated ageing characteristics. When exposed to UVB (in vitro), skin cell types like fibroblasts and keratinocytes show DNA damage and increased senescence markers, such as increased SASPs. [3] Dermal fibroblasts also release insulin-like growth factor (IGF)-1, essential for epidermal cell proliferation and differentiation. [5] IGF-1 signalling in senescent fibroblasts is significantly decreased [6]. Inhibition of the IGF-1 pathway decreases collagen production in the dermis, causing epidermal thinning. Additionally, mitochondrial dysfunction and increased levels of superoxide anions prompt fibroblast ageing, thereby speeding up the skin ageing process. [5] Fibroblasts isolated from photo-aged skin produce a greater amount of pro-melanogenic growth factors. [14] Ageing-associated pigmentation has also been reported to be driven by (UVA-induced) fibroblast senescence. [15-16] Keratinocyte senescence The epidermis shows less impact of senescent keratinocytes due to their quicker turnover in comparison to fibroblasts. Senescent keratinocytes experience reduced ECM production and cell adhesions [8], along with elevated MMP expression in UV-induced senescence [9], and increased SASP levels, including pro-inflammatory cytokines. [10] Airborn particulate matter (PM2.5) can penetrate a disrupted skin barrier. PM2.5-induced ROS leads to epigenetic modification: reduced DNA methyltransferase, elevated DNA demethylase expression, p16INK4a promotor hypomethylation and therewith accelerated keratinocyte senescence. [11] Keratinocytes are the main type of cells that signal the need for melanogenesis. [12] UVR-induced DNA damage in keratinocytes activates melanogenesis. [13] Melanocyte senescence Senescent melanocytes express markers of inflammageing and dysfunctional telomeres. Senescent melanocyte SASPs induce telomere dysfunction and limit the proliferation of the surrounding cells, hence, senescent melanocytes affect and impair basal keratinocyte proliferation and contribute to epidermal atrophy. [17] STRATEGIES TO COMBAT CELL SENESCENCE PREVENTION Sunscreen: Protection against UV radiation combined with blue light defense (Licochalcone A: powerful anti-oxidant, Nrf2-Activator & increasing Glutathione + Colour pigments) and prevention + repair DNA damage (Glycyrrhetinic Acid) INTERVENTION Senotherapeutics can be classified into three development strategies: [25]
Skin care ingredients: [18]
Of course a healthy life-style and diet (consider also intermittent fasting) will support both your body & skin longevity and beauty Prevention and intervention of skin cell senescence offers a promising approach to improve skin health and beauty. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Many of the skin regenerating or rejuvenating treatments, like energy based devices in the doctors-office are based on the principle to cause controlled damage and therewith provocation of a skin rejuvenating repair response. One of the fascinating mechanisms behind laser "damage" is the heat shock response leading to increased production of regenerating heat shock proteins (HSPs). Heat shock proteins respond to heat stress, are crucial cellular defense mechanisms against stress (environmental and physiological), act as chaperones, aiding in protein folding, prevention of protein damage, cellular protection and repair. [1]
HEAT SHOCK PROTEINS AND OX-INFLAMMAGEING UV radiation and blue light cause oxidative stress and inflammation, and can overwhelm skin's own capacity to counteract the increased formation of reactive oxygen species (ROS) and inflammatory mediators. Chronic oxidative stress state and chronic low grade of inflammation are hallmarks of skin ageing and their combination can be called ox-inflammageing. Oxidative stress and inflammation alter cellular signal transduction pathways and thereby the expression of the ECM genes as well as the structure of the ECM proteins like collagen, fibronectin and elastin. Their reduced expression and increased degradation manifests eventually at the skin surface as wrinkles, loss of firmness, and elasticity. Heat shock proteins are chaperone proteins that facilitate the formation of the ECM and prevention of molecular oxidative damage or degradation and are classified based on their molecular weights.
STIMULATION OF REJUVENATING HEAT SHOCK PROTEINS Heat shock protein synthesis can be initiated not only by heat but also by many chemical and physical stimuli, such as heavy metals, amino acid analogues, oxidative stress, viral infection and UV and ionizing irradiation. [10] Laser Laser treatments have been shown to induce a heat shock response in the skin from epithelial cells to deeper connective tissues, leading to the production of heat shock proteins. This response is characterized by the temporary changes in cellular metabolism, release of growth factors, and increased cell proliferation and thus contribute to tissue regeneration and rejuvenation. [11] CBD It has been proven that a large number of genes belonging to the heat shock protein super-family were up-regulated following cannabidiol (CBD) treatment. [12] UV radiation Ultraviolet radiation (UV)‐induced cell death and sunburn cell formation can be inhibited by previous heat shock exposure and UV itself can induce HSP expression. However, levels of HSP-27 have been found to be elevated in sun‐protected aged skin indicating a link between HSP-27 expression and age‐dependent epidermal alterations. [13] I would recommend daily protection from UV radiation and blue light (or high energy visible light). Ultrasound Ultrasound exposure at different frequencies, intensities, and exposure times can induce HSP-72 expression. Higher ultrasound frequencies, such as 10 MHz, have been found to significantly increase HSP-72 levels. Additionally, increasing the temperature during ultrasound exposure has shown to enhance HSP-72 expression. Interestingly, ultrasound at 1 MHz was unable to induce HSP-72 significantly, while 10 MHz ultrasound induced HSP-72 after 5 minutes of exposure. [10] Radiofrequency Radiofrequency has been shown to increase HSP-70 and decrease melanin synthesis and tyrosinase activity. [14] RF-US treatment significantly increased levels of HSP47 proteins. [15] Red & near infra red light Although I've not seen much peer reviewed published evidence, red light and near infra red light therapy may release the HSPs in the skin if tissue reaches >42 - 45 degrees (even for 8 - 10 seconds). Nicotinamide Nicotinamide and its derivatives have been found to stimulate the expression of heat shock proteins, including HSP-27, HSP-47, HSP-70, and HSP-90 in the skin. These proteins play as mentioned before an essential role in collagen production, skin protection, skin health and rejuvenation. [6] NAD as nutrient interestingly has proven to tweak the epigenome by modulating DNMT1 enzymatic DNA methylation and cell differentiation. [16] In topical applications an ingredient called Dihydromyricetin also called Epicelline® has been successful in inhibiting DNMT1 enzyme activity biochemical assays. [17] Stimulation of heat shock proteins offers a promising and novel invasive, non invasive and topical approach for skin regeneration, rejuvenation and reduction of ox-inflammageing. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Like epigenetics and exosomes, neurocosmetics represent a revolutionary approach for skin care incorporating neuroscience principles, leveraging the skin-brain connection to improve skin health and beauty. The term itself is a fusion of the words neuroscience and cosmetics. It differs from psychodermatology which like neurocosmetics connects the interaction between mind and skin, but in a different way. Some describe it as how simple sensory stimulation can improve our overall wellbeing and call it "mood beauty", however this doesn't do it justice as neurocosmetics go beyond mood boosting skincare.
DEFINITION NEUROCOSMETICS Dermatologist Professor Laurent Misery back in 2002 described that neurocosmetics are products which are supposed to modulate the neuro-immuno-cutaneous-system (NICS) function at an epidermal level. Skin cells can produce neuromediators, which are mediators for transmission of information between skin, immune and the nervous system. All skin cells express specific receptors for neuromediators and by binding of the neuromediator to its receptor, modulation of cell properties and skin functions are induced like cell differentiation and proliferation (renewal), pigmentation, etc. Hence, keratinocytes, Langerhans cells, melanocytes, endothelial cells, fibroblasts and the other cells of the skin are modulated and controlled by the nerves and in return skin is able to modulate neuronal activity and growth. [1] SKIN-BRAIN CONNECTION In an article from the International Journal of Novel Research and Developments, the skin-brain connection was described as a psychobiological concept that highlights how emotions, stress, and neurotransmitters impact skin health. Indicating that the skin acts as a neuroimmunoendocrine organ, emphasizing its sensitivity to neural signals and stress responses. [4] CUTANEOUS NERVOUS SYSTEM The skin a sophisticated sensory organ that allows you to interact with your environment through touch and feel. It contains a complex network of nerves that send information about sensations like pressure, pain, itch and temperature from the skin through the spinal cord to the brain [9]. The dynamic interactions between the skin and the nervous system is influenced by factors like stress and inflammation, which can impact skin health and ageing. [7] Nerves in the skin: These nerves are like tiny messengers that tell your brain about what your skin is feeling: pressure, heat or pain. Types of nerve fibers: Some are thick and wrapped in a protective coating, which helps them send messages quickly. Others are thin and slow but are very good at sending messages about pain or temperature changes. [3] Sensory receptors: These receptors can tell if something is touching the skin lightly or if there's a lot of pressure. They can also sense if something is hot, cold, or causing pain. [3] Autonomic nervous system: Part of the cutaneous nervous system helps control things that happen in the skin automatically, like sweating to regulate body temperature. [8] Nerve cells: There are about 20 different types of neurons in our skin. [10] The contribution of epidermal keratinocytes to NICS [3]
CUTANEOUS NEURO-AGEING Neuro-ageing is defined as the changes in the nervous system which cause continuous neurodegeneration due to oxidative stress, neuroinflammation or impaired neuromodulation. As skin ages, Aβ-toxin (increased by oxidative stress) accumulates at the nerve endings innervating the tissue, causing disrupted cellular communication, particularly affecting fibroblasts’ ability to produce collagen and extracellular matrix. On top there is a decrease of nerve growth factor (NGF) production, important for the development and maintenance of nerve cells. Different factors can lead to a drop in NGF production, resulting in malfunctioning keratinocytes and reduced lipolytic activity of adipocytes, visibly impacting skin hydration and firmness. [6] Skin nerve fibres are significantly reduced in number following UV irradiation and in ageing skin [5] and therefore neuro-protectors or targetting neurodegeneration can reduce stress manifestations and promote healthy cellular communication for optimal skin function. [3] Although not much is known regarding skin specific or topical neuroprotectors (most research was focussed on the brain), probably potent anti-oxidants, by significantly reducing oxidative stress from UV and blue light and anti-inflammatory ingredients may inhibit skin neuro-ageing and can be neuroprotective especially when combined with sunscreen and strengthening of the skin barrier. NEUROCOSMETIC VARIETY OF ACTIONS
THE FUTURE OF NEUROCOSMETICS The neurocosmetics market is booming, with a projected value of USD 2.69 billion by 2030. [11] The future of neurocosmetics holds promise for innovative ingredients and concepts that harness new neuroscientific insights to revolutionize skin care and sunscreen formulations, to cater to both physical and emotional aspects of skin health and beauty. Take care! Anne-Marie References

One of the people I follow ever since I started to work on skin epigenetics back in 2017 and longevity is Harvard professor David Sinclair. He is best known for his work on understanding why we age and how to slow its effects. He was talking about hormesis, a phenomenon where exposure to low doses of stressors induces beneficial effects. A hormetic (cellular defense) response can modulate ageing processes by activating genes related to maintenance and repair pathways through mild stress exposure in our body and skin, leading to enhanced longevity (thus anti-ageing) and health. [1 - 2]
Originating from the early 2000s, the concept of hormesis has evolved to evidenced based dermatological applications. [3] Various factors, including environmental stressors, lifestyle choices, and genetic predispositions, can influence the hormetic responses in skin cells. Understanding these influences is essential for optimizing skin health and beauty through hormetic pathways. Many terms are used for hormetic responses in the scientific literature, including the Arndt-Schulz Law, biphasic dose response, U-shaped dose response, preconditioning/adaptive response, overcompensation responses, rebound effect, repeat bout effect, steeling effect, among others. [4] Ageing is an emergent, epigenetic and a meta-phenomenon, not controlled by a single mechanism. Cellular damage has three primary sources: [3]
Effective homeodynamic space or buffering capacity (body's ability to maintain stability or balance in changing conditions) is characterized by:
Stress response is a reaction to physical, chemical, or biological factors (stressors) aimed at counteracting, adapting, and surviving, is a critical component of the homeodynamic space. There are seven main cellular stress response pathways:
Hormetins can be categorized into three types:
Being aware of the phenomenon of hormesis can result in discovering the usefulness of new compounds, or synergistic effects of combining hormetic treatments which otherwise may have been rejected due to their effects of stress induction. What is bad for us in excess, can be beneficial in moderation, or (quote): "What doesn't kill you makes you stronger". [6]. The future of hormesis in dermatology holds great promise for innovative interventions, advanced hormetic technologies or personalized skin care regimens. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie Read more: The impact of senescent zombie cells on skin ageing The role of heat shock proteins in skin rejuvenation Neurocosmetics, the skin-brain connection & neuro-ageing The role of the lymphatic system in ageing skin The power of light and photo-biomodulation Bio-stimulators Skin glycation Exosomes References

While factors like genetics and lifestyle (including sun exposure) play significant roles in skin ageing, the role of the lymphatic system in skin ageing is an overlooked however interesting strategy to improve skin's youthful functional (health) and physical attributes (beauty).
The lymphatic system, a vital part of the immune system, is responsible for draining excess fluid, toxins, and waste products from tissues. In the skin, lymphatic vessels collect waste and transport it to lymph nodes for filtration. The lymphatic vessels work with tiny, reflexive muscular contractions constantly pumping cleansing (toxins and debris) lymph fluid through their channels. Interestingly it explains why injections with the muscle relaxant botulinum toxin can cause oedema. The function of the lymphatic system
As we age the lymphatic function and density is decreasing 1:
Effects of lymphatic system decline on skin:

Rejuvenating the lymphatic system for youthful sculpted skin:
Wrongful injected fillers in the tear trough or malar (eye socket - cheek area) septum can lead to worsening of malar oedema (fluid retention) or malar bags. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References: 1. Structural and Functional Changes in Aged Skin Lymphatic Vessels R. Kataru et al. Front. Aging, 2022 2. Reduction of lymphatic vessels in photodamaged human skin Kentaro Kajiya, Rainer Kunstfeld, Michael Detmar, Jin Ho Chung J Dermatol Sci. 2007 3. Patent Cosmetic preparations comprising natural activators 4. Patent Cosmetic preparations comprising daphne extracts 
If you've scrolled through Instagram, you may have caught a glimpse of dermatologists raving about LED masks emitting red light, the secret, evidenced based weapon behind skin rejuvenation known as photo biomodulation. It uses low-powered light within the red to near-infrared range (wavelengths from 632 to 1064 nm) to induce a biological reaction aka stimulate cellular processes. The wonders of red light, also known as LLLT (low-level laser therapy), PBM (red light photo-biomodulation), or PBMT (photo-biomodulating therapy), extend far beyond non-invasive skin rejuvenation. I am not a fan of devices for home use, mostly because of lacking safety and/or efficacy, PBM definitely earned it's prominent spot in my skincare routine.
A summary of the benefts of red light with and without near infrared light for skin Numerous studies have demonstrated the effectiveness of red and infrared light therapy for skin rejuvenation. A combination of red light and near IR light has proven to stimulate the production of collagen (I & III) plus elastin production (Li WH et al Int J Cosmet Sci 2021), enhance mitochondrial ATP production, cell signaling, growth factor synthesis, rebalance ROS (reactive oxidative species) and reduce inflammation. Stem cells can be activated allowing tissue repair and healing. Wrinkle and scar reduction was observed and it can reduce UV damage both as treatment and prophylactic measure. In pigmentary disorders such as vitiligo, it can increase pigmentation by melanocyte proliferation and reduce depigmentation by inhibiting autoimmunity (Pinar Avci et al. Semin Cutan Med Surg. 2013 & Mitchell J Winkie et al. Review Photodermatol Photoimmunol Photomed A focused review of visible light therapies for vitiligo 2024). It has the potential to activate both keratinocytes (epidermis) and fibroblasts (epidermal junction and dermis). With consistent use, you can expect a reduction of lines and wrinkles, improvement of skin tone and texture. PBMT (when done effective and safe) will compliment both your skin rejuvenating and regenerating at home skincare regimen and in-office procedures or even post-surgical skin recovery. ATP ATP (adenosine triphosphate) is the primary source of energy for cellular processes and plays a crucial role in various biological functions. When red light with specific wavelengths (630 nm to 638 nm and 810 nm) is absorbed by the skin cells, it stimulates the mitochondria, which are the powerhouses of the cells responsible for ATP synthesis. This increase in ATP production is providing cells with more energy to carry out their functions effectively and has several beneficial effects on the skin like boosting cellular metabolism, promoting more efficient nutrient uptake and waste removal. The increased ATP levels facilitate collagen synthesis by fibroblasts, a vital component for skin structure, elasticity and firmness and reduction of lines and wrinkles.. ATP aids in the repair and regeneration of damaged skin cells. It accelerates the healing process, making it beneficial for wound healing, post-surgical recovery, and addressing skin issues such as acne scars. ROS (Reactive Oxidative Species) By modulating ROS levels, red light therapy helps reduce oxidative stress and its detrimental effects on the skin. ROS are highly reactive molecules that are naturally produced by cells as byproducts of metabolic processes. While low levels of ROS play important roles in cellular signaling and immune responses, excessive ROS can lead to oxidative stress and damage to cells and tissues. Restoring the balance of ROS result in improved skin health, reduced inflammation, and enhanced skin rejuvenation. Red light therapy has been shown to modulate reactive oxidative species (ROS) levels in the skin by promoting antioxidant defense mechanisms and reducing oxidative stress:
The difference between LLLT and PBM LLLT refers specifically to the use of lasers, which produce coherent, focussed and an intense beam of monochromatic light, while PBM has a broader range of light sources, may include laser as well as light-emitting diodes (LEDs) and other non-laser devices. LEDs are often used in PBM because they are cost effective, versatile and have the ability to cover large treatment areas. LLT uses higher power densities with more energy and has a shorter treatment duration in comparison to PBM to achieve desired therapeutic effects. While there are similarities in terms of mode of action", there is a difference of light source, treatment application and parameters. Based on consensus, PBM and PBMT are considered the correct way to describe this photonic specialty for therapeutic applications. In this post I will focus on PBM and specifically LEDs. LED masks and LED panels LED masks specifically produced by the brand Omnilux (FDA cleared) are currently very popular for very good reasons; they are safe and effective when the LEDs emit the right wavelengths and used in the recommended frequency. Omnilux combines 2 therapeutically effective and complimentary wavelengths: 633nm and near-infrared 830 nm. Both wavelengths (more precise 630nm + 850nm) I would recommend to minimally look for in any red LED device, which will disqualify most LED masks and panels in the market! I've include some (not affiliated) links to devices below. Both masks and panels can be effective, however most panels are stronger in comparison to masks 60 mW/cm² vs mW/cm²), hence have the benefit of a shorter treatment time to get a similar result. Intensity and power of red light therapy devices are typically measured in terms of irradiance (measured in milliwatts per square centimeter, mW/cm²) and radiant flux (measured in watts, W), which quantify the amount of light energy emitted by the device. Wearing a mask during a hot summer or in a warmer climate will make you sweat and depending on the materials of the mask and straps, they may be very uncomfortable to wear. Panels have the benefit that they give a more even distribution of emitted light as masks are worn on the face and thus the LED bulbs are pushed on a small skin surface area, panels can cover a larger area (depending on their size) and are more versatile in use, as area's like neck, décolletage, or knees are easier to treat with a panel. With a mask you may be more mobile, although I would not recommend walking around while using the mask. My personal preference would be a panel for the reasons mentioned before and panels are more suitable (more hygienic) for family sharing. My son can use it after an intense workout to speed up his recovery and I like to use it for purposes beyond photo-biomodulation or skin rejuvenation, for example to improve my sleep. With a panel I get more "bang for my buck". 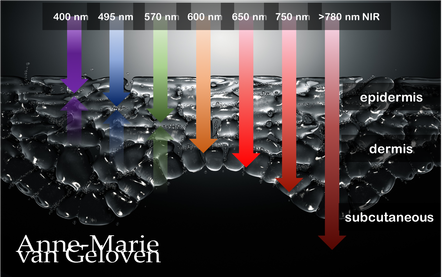
Red light and NIR (Near Infra Red light) have the ability to penetrate varying depths of the skin, resulting in distinct benefits, thus combinations of wavelengths will provide complementary effects.
630 nm Wavelength This wavelength is often used for its skin rejuvenation benefits. It has a relatively shallow penetration depth and is absorbed closer to the surface of the skin primarily affecting the epidermis. 630nm light is associated with increased circulation, reduce inflammation, improved skin tone & texture, aiding in the delivery of nutrients and oxygen to skin cells, and stimulating the production of collagen, leading to improved skin elasticity and a reduction of the appearance of fine lines & wrinkles. 660 nm Wavelength At 660nm, red light can penetrate a little deeper into the skin, reaching the dermis. It is known for its ability to stimulate collagen production, enhance cellular metabolism, and promote anti-inflammatory effects, helping to reduce redness and inflammageing. It also promotes wound healing, making it beneficial for post-surgical or post-trauma skin recovery. 810 nm Wavelength Improve healing & recovery & accelerate wound healing. 830 nm Wavelength Accelerate healing, reduce infection, improve aesthetic outcome following plastic surgery, increase endorfines (mood-enhancing), improve bone repair and growth. 850 nm Wavelength Improve general inflammation body, enhance muscle recovery, improve wound healing, reduced fine lines, wrinkles and hyperpigmentation. Always consult a qualified healthcare professional or dermatologist to determine if and what the most suitable red light therapy approach is for your particular skin condition and rejuvenation goals. Take care! References: Hamblin, Michael R. "Mechanisms and applications of the anti-inflammatory effects of photobiomodulation." AIMS biophysics 4.3 (2017): 337-361. Barolet, Daniel. Regulation of Skin Collagen Metabolism In Vitro Using a Pulsed 660 nm LED Light Source: Clinical Correlation with a Single-Blinded August 2009Journal of Investigative Dermatology 129(12):2751-9 Wunsch A, Matuschka K. (2014). A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Journal of Cosmetic and Laser Therapy, 16(5), 232-237. Avci P, et al. (2013). Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Seminars in Cutaneous Medicine and Surgery, 32(1), 41-52. Links to some devices which combine 630 nm and 850 nm: FDA-approved devices ensure safety and regulatory compliance, however the panels are more powerful: Omnilux(tm) Mask (FDA clearance) Very affordable panel (no FDA clearance) Affordable panel (no FDA clearance) 5/11/2023 Comments The right amount of skin care
Using the right amount of a skin care product is as important as picking the right product(s). If you don't apply enough of the product or for a too short duration, you will not get the optimal result. This is particularly crucial when using sunscreen to reach the sufficient SPF level and protection. According to a study published in the Journal of the American Academy of Dermatology by Andreas Storm MD et al. 95% of patients with a topical treatment under-dose, hence do not use enough cream.
If there is a specific user manual mentioning the dosage, or you got a prescription, follow their recommended instructions. If the product came without specific dosage instructions, there is a general rule of thumb. The recommended amount of product to apply varies, depending on the product type. THE 2 FINGERS RULE FOR SUNSCREEN For sunscreen you need 1/2 teaspoon for the face or enough to cover the bottom of a shot glass and a full shot glass for the body, which should add up to 2mg per cm2. Another method is using the "rule of nines, which is used for burns. The body areas are divided into 11 area's, each representing 9% of the total. Sunscreen can be applied to each of these areas at a dose of 2 mg/cm2 (regardless phototype) if two strips of sunscreen are squeezed out on to both the index and middle fingers from the palmar crease to the fingertips, thus 2 fingers. (1) The body areas are: 1 Head, neck, and face 2 Left arm 3 Right arm 4 Upper back 5 Lower back 6 Upper front torso 7 Lower front torso 8 Left upper leg and thigh 9 Right upper leg and thigh 10 Left lower leg and foot 11 Right lower leg and foot FINGERTIP UNITS For the use of other topical products there is a guidance created called Finger Tip Units or FTU's by CC Long and AY Finlay. It is a way of measuring the amount of product squeezed out of a tube with a 5mm diameter nozzle and applied from the distal skin-crease (the crease closed to the fingertip) to the tip of the index finger. The FTU concept has been used as a central part of an education programme for parents of children with atopic eczema, has been advocated to reduce the variation in usage of topical steroids and to encourage adherence to therapy. For a serum, you may need less as they are lightweight products which should be fully "absorbed" without residue. If the skin still feels sticky after 1 minute, you probably applied too much product. A guidance would be a pea size dot on forehead, right cheek, and left cheek, which is similar to the recommended amount of retinoids (Vitamin A). However, unlike Vitamin A, using too much serum usually isn't harmful for the skin, but increases the risk of "pilling". 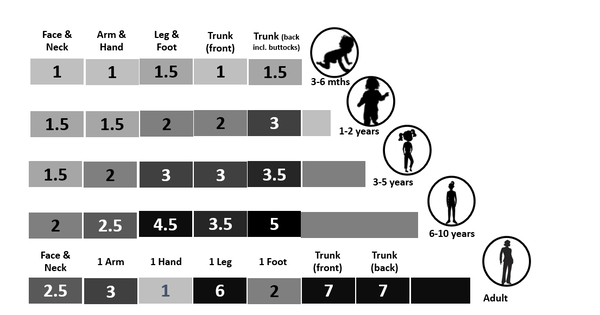
The precise number of FTU's required:
One FTU covers 286 cm2, more specifically in males and 312 cm2 in females 257 cm2. The quantity of cream in a fingertip unit varies: Adult male: 1 fingertip unit provides 0.5 g Adult female: 1 fingertip unit provides 0.4 g . Keep in mind this is a general guideline and the amount of product needed or results may vary also depending on skin type, concerns and the products particular attributes. Take care (in the right amount and duration) References: 1. BMJ. 2002 Jun 22; 324(7352): 1526.Simple dosage guide for suncreams will help users Steve Taylor et al. Illustration Tinea incognito with unjustified use of potent Topical Corticosteroids: a case series July 2017 International Journal of Basic & Clinical Pharmacology 6(8):2087 Haiya Sheth et al. 
Skin ageing is a biological degenerative process, marked by loss. The number of patients seeking nonsurgical rejuvenation of the face and the body is continuing to increase due to a growing ageing population concerned with physical appearance. Women wish to maintain a youthful appearance and attractiveness represent 92% of all cosmetic procedures.(1) Men are keen to maintain physical characteristics associated with virility.(2) Millennials are also increasingly concerned with preserving their beauty and youth.(3) Among the various treatment approaches, different minimally invasive techniques have been developed and dermal fillers currently come second after botulinum toxin type A (BTA).(3) Their use is increasing worldwide.
"The fear of looking done is the number 1 reason why patients don't seek treatment"* The range of fillers available for soft-tissue augmentation is constantly expanding. The latest advances in filler technology include bio-stimulators that exert their aesthetic effect by promoting predominantly collagenesis or biological stimulation of new collagen and sometimes also elastin production. Therewith they provide a biological answer to the skin ageing degeneration process, with gradual and often very natural results. Over the course of last years the knowledge on injectable bio-stimulators has grown, and therewith their safety and popularity as they provide subtle longer lasting results. Facial fillers can be broken into 3 main groups:
Bio-stimulating fillers promote the body’s natural production of some ECM components (mostly collagen) over a period of several months. Their differences are characterized by their property of inducing natural collagen production. SYNTHETIC BIOSTIMULATORS
Calcium Hydroxylapatite Calcium hydroxylapatite: Calcium hydroxylapatite is a type of mineral that is commonly found in human teeth and bones and in injectbales the calcium hydroxylapatite particles are suspended in a gel-like solution. The effects of this material last approximately 18 months with minimal inflammatory response. Radiesse is a biodegradable filler consisting of 30% synthetic CaHA microspheres (diameter of 25-45μm) suspended in a 70% aqueous carboxymethylcellulose gel carrier. The soluble carrier gel evenly distributes the Radiesse CaHA microspheres providing 1:1 correction and gradually dissipates leaving the microspheres at the injection site where they induce collagenesis (collagen type I and mostly collagen type III) by fibroblast activation. Animal studies have shown that this new collagen growth occurs as early as four weeks post-injection and continues for at least 12 months with an average duration of effect of 12 to 18 months, though some results have been noted 24 months post-injection. Radiesse provides both immediate (replacement volume) and long-lasting (collagen biostimulation) volume enhancement. (5) Poly-L-lactic acid PLLA is a biodegradable, bioresorbable biocompatible man-made polymer. This material has wide uses in absorbable stitches and bone screws. The effects of PLLA generally become increasingly apparent over time (over a period of several weeks) and its effects may last up to 2 years. There is an inflammatory response. PLLA is an alpha hydroxy acid polymer of the lactic acid L-enantiomeric structure that has been safely used in many applications and in medicine for more than 30 years. Its use has expanded worldwide, associated with good long-term aesthetic results thanks to its biostimulatory-collagen effect. PLLA-based fillers are supplied as a lyophilized powder to be reconstituted with sterile water. The collagen stimulatory properties were evidenced in human in subjects (n=14) who received PLLA injections (3 sessions, spaced 4 weeks apart) at the postauricular level by collagen histochemical determination on biopsies taken at different times. Increase of collagen type-I was shown at 3 and 6 months. This study opened the new class of collagen stimulators. The long duration of action was demonstrated in a first pivotal study comparing PLLA versus collagen (116/117 subjects, respectively); the long-term safety/efficacy was shown up to 25 months. The rationale for several sessions was first documented in a dedicated article; this modality allows the effect through collagen stimulation, a biological process to occur and avoids overcorrection. PLLA fillers are among the most clinically documented products. (6) Polymers, polycaprolactone The PCL-based collagen stimulator is composed of PCL microspheres suspended in a carboxymethyl-cellulose gel carrier providing immediate and sustained volumizing effects when injected; the morphology, the biocompatibility of the PCL microspheres embedded with the collagen fibers produced all contribute to the creation of a unique 3D scaffold for a sustained effect. Its safety has been investigated in clinical studies and vigilance surveys. It presents the advantage of a slower degradation than polylactic acid (PLLA) or polyglycolic acid (PGA), which both belong to the same chemical family. Both the S and M products induced collagen production. In animal, the M product induced collagen type-III and type-I at early stage (measure at 9 months), and later predominantly collagen type-I, that deposits around the PCL microspheres (measure at 21 months). Many fibroblasts were found near the PCL microspheres. Interestingly, new elastin fibers were also formed, and neovascularization with new capillaries observed as well. (7) NATURAL BIOSTIMULATORS 1. Platelet rich plasma 2. Platelet rich fibrin 3. Polynucleotides like Nucleofill or Nucleadyn 4. Exosomes 5. Alginate 6. Tropoelastin (precursor of elastin molecule) 7. Poly-y-glutamic acid Platelet-Rich Plasma (PRP): PRP treatments are produced by spinning a small volume of the patient’s own blood through a centrifuge. This separates and concentrates the blood’s components, including platelet-rich plasma and the “buffy coat,” a solution that contains immune cells. The provider combines these two components with a small amount of calcium chloride (which activates and keeps the PRP stable), then injects them into the treatment area. Over a period of months, PRP stimulates the body’s natural collagen production. Platelet-Rich Fibrin (PRF): PRF is produced using a process similar to PRP concentration. The active material is a fibrin matrix rich in platelets, stem cells, and immune cells. Like PRP, PRF treatment stimulates collagen production and is also implicated in tissue regeneration, though there’s less data on the durability of its effects. Because both treatments use material from the patient’s own body, so there’s no risk of rejection or similar complications. PRF and PRP effects are durable — typically lasting longer than 18 months. Polynucleotides: Polynucleotides are most often natural, highly purified DNA molecules extracted for example from trout gonads and activate specialised cells called myofibroblasts and adipocytes. PN containing devices act as short time temporary fillers thanks to the viscoelasticity of the long DNA fragments and improve skin well‐being (cell growth) and steady self‐repair (tissue regeneration). Read more Exosomes: The use of exosomes at the Aesthetic & Anti-Aging Medicine World Congress in Monaco was discussed during many session and some excellent results were presented. However their use is not yet approved and safety and long-term effect not yet established and largely depends on the source. Read more BOTULINUM TOXIN There is evidence that the neuromodulator or musclerelaxer Botinumtoxin after injection upregulated the expression of type I collagen, decreases the production of some MMPs in fibroblasts, preventing collagen degradation and improves collagen organisation. (8.9.) ENERGY BASED DEVICES Intense Pulsed Light/BroadBand Light, Radiofrequency Microneedling, lasers, High-Frequency Ultrasound, Electromagnetic Tec. stimulate collagen production via a controlled damage and repair mechanism. DERMO-COSMETICS WITH BIO-ACTIVES There are innovative dermo-cosmetic products containing bio-stimulating ingredients, working more superficial in comparison to in-office treatments and they therefor are potentially an excellent choice as adjunctive care for biological rejuvenation and revitalization for younger looking and acting skin. They are safe to use easy to apply over face, neck and décolletage. Unlike in-office treatments their effects are temporary (fully reversible as regulated), hence they require daily or twice daily application. Bio-stimulating active ingredients in skincare which have shown to particularly stimulate the fibroblast are for example:
VITAMIN C IS NEEDED FOR COLLAGEN SYNTHESES! Our skin needs Vitamin C to produce collagen and is not able to produce it, thus relies on external resources for supply. Therefore I highly recommend to either get enough Vitamin C from your diet or use a high quality topical product pre & post biostimulators. Read more As the biological degeneration takes place in different layers of the skin and it's underlying structures, combining in-office treatments specifically targeting those layers in a series of treatments may provide longer lasting results and give higher patient satisfaction.(13) Safety and outcome rely on the qualification and experience of your cosmetic doctor, dermatologist or plastic surgeon. Take care Special thanks MD FAAD Hassan Galadari Jair Mauricio Cerón Bohórquez M.D. References: 1. American Society Plastic Surgeons. 2020 national plastic surgery statistics; 2020. 2. Wat H, Wu DC, Goldman MP. Noninvasive body contouring: a male perspective. Dermatol Clin. 2018;36(1):49–55. 3. Wang JV, Akintilo L, Geronemus RG. Growth of cosmetic procedures in millennials: a 4.5-year clinical review. J Cosmet Dermatol. 2020;19(12):3210–3212. 4. Evaluation of the biostimulatory effects and the level of neocollagenesis of dermal fillers: a review. Haddad S, Galadari H, Patil A, Goldust M, Al Salam S, Guida S International Journal of Dermatology, 29 Apr 2022 5. J Clin Aesthet Dermatol. 2015 Jan; 8(1): 38–49. Calcium Hydroxylapatite Over a Decade of Clinical Experience Jani Van Loghem, MD, Yana Alexandrovna Yutskovskaya, MD,b and WM. Philip Werschler, MDc 6. Clin Cosmet Investig Dermatol. 2022; 15: 997–1019. Collagen Stimulators in Body Applications: A Review Focused on Poly-L-Lactic Acid (PLLA) Marie-Odile Christen Read more 7. Clin Cosmet Investig Dermatol. 2020; 13: 31–48. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics Marie-Odile Christen and Franco Vercesi 8. Oh SH, Lee Y, Seo YJ, Lee JH, Yang JD, Chung HY, Cho BC. The potential effect of botulinum toxin type A on human dermal fibroblasts: an in vitro study. Dermatol Surg. 2012 Oct;38(10):1689-94. 9. El-Domyati M, Attia SK, El-Sawy AE, Moftah NH, Nasif GA, Medhat W, Marwan B. The use of Botulinum toxin-a injection for facial wrinkles: a histological and immunohistochemical evaluation. J Cosmet Dermatol. 2015 Jun;14(2):140-4 10 EADV 2022 Inhibition of extracellular matrix degrading enzymes and bio-stimulation of fibroblasts – A novel approach to mitigate the advanced degenerative process in skin aging Weise J, Vogelsang A, Sperling G, Welge V, Nölter A, Mielke H, Knott A, Harbig S, Stuhr A, Dunckel J, Warnke K, Geloven van A 11. EADV 2021 Multifaceted novel approach to increase skin’s own epidermal and dermal hyaluron content Bussmann T, Warnke K, Krüger A, Möller N, Harbig S, Stuhr A, Dunckel J, Geloven van A, Weise J | Beiersdorf AG, Hamburg, Germany 12. Photochemistry and Photobiology, 2005, 81: 581–587 Novel Aspects of Intrinsic and Extrinsic Aging of Human Skin: Beneficial Effects of Soy Extract Kirstin M. Su¨del et al 13. Combination Therapy in Midfacial Rejuvenation Humphrey et al. Dermatologic Surgery 42:p S83-S88, May 2016. *AMWC 2023 Tapan Patel 5/6/2023 Comments The impact of humidity on skin
Something I am asked quite regularly is if low humidity can dry out the skin. The answer is yes it can. However, it really depends on your skin. There are 4 skin types: normal, dry, combination and oily. According to the (AAD American Academy of Dermatology) sensitive skin is a skin type too, however some would say all skin is sensitive and I somewhat agree. Dehydrated skin is not a skin type, but a (temporary) condition. Your skin type is pretty much set for life and not changing with the seasons. There is an exception as normal, oily or combination skin may become dry(er) post-menopause. The environment, including temperatures and humidity impact our skin significantly.
Humidity Humidity is the amount of water vapor in the air. If there is a lot of water vapor in the air, the humidity will be high. A relative humidity of 70 percent means the air is at 70 percent of its water-holding capacity for the present temperature. Cold air cannot hold as much water vapor as warm air. Thus, as temperature falls, with no change in the amount of water in the air, the relative humidity rises. HIGH HUMIDITY A study showed that in a dry environment the skin hydration decreases but the amount of sebum increases to compensate for skin dryness. (1) High humidity can be beneficial if you have dry skin, however can cause problems if you have oily or combination skin. Low levels of humidity can negatively affect your skin, even when your skin is oily. In general high humidity levels has some benefits. Hydration The increased levels of moisture in the air in high humidity decrease trans-epidermal-water loss of water evaporation from the skin. Hence, your skin is able to maintain it's hydration levels. Increased cell regeneration A moisture-rich climate can also promote skin cell turnover and desquamation (the shedding of dead skin cells). Skin’s regeneration process is increased when your skin is well hydrated. The shedding of dead skin cells is the last step in this cell-turnover or regeneration process and good desquamation leads to smoother texture and more radiant skin. Well-ageing effects Fine lines and wrinkles are more noticeable if your skin lacks moisture and feels dry. Moreover, skin regeneration process declines as we age. Since high humidity positively contributes to hydration, lines and wrinkles are less pronounced and the regeneration process supported, therewith leading to more youthful looking skin. Increased sebum production If your skin is oily, high humidity (especially heat thus sweat) can increase sebum production. An overproduction of sebum, especially in combination or oily skin types can make your skin oily and greasy. Moreover, dirt and irritating particles may "stick" better to greasy skin. Acne prone skin Excess sebum and oil caused by high humidity (heat and sweat) increases the risk of clogged pores, break-outs and comedones (blackheads, and whiteheads). Heat or sweat rash Heat rash, or sometimes called prickly heat, sweat rash or miliaria, is a harmless but very itchy or prickling skin rash. It causes small red (raised) spots in places where sweat collects, such as the armpits, back, under the breasts, chest, groin, elbow creases and back of the knees, and the waist. Although uncomfortable, it is usually harmless and improves on its own after a few days. Tips to take care of your skin in humid conditions
LOW HUMIDITY Low levels of humidity most commonly negatively affect your skin. Decrease skin regeneration When your skin is not well hydrated, the skin's regeneration process including shedding of dead skin cells is impaired. This can lead to a more dull, rough or even flaky skin. Dehydrated skin Your skin can get dehydrated or deprived of moisture when exposed to low humidity levels and the skin looks less radiant or glowing. Sings of ageing When your skin lacks moisture, the skin is less plump and wrinkles, fine lines become more visible. Worsening of skin conditions If you have eczema or another problematic impaired barrier related skin condition, low humidity can cause flare-ups. It can also cause or worsen dry skin symptoms like redness, scaliness, rough texture, cracks, itchiness, stinging, burning and the skin might be more prone to irritation and infection. Tips to care care of your skin in low humidity
Take care References

Skin complaints related to visual display terminals (VDTs) such as smartphones, tablets, and computers are on the rise. Visually it's a rosacea-like dermatitis (erythema, oedema, papules or pustules), and can be accompanied by itch, pain and smarting (1). Some only have subjective symptoms and no visible skin problems.
It was thought that this skin condition might be caused by prolonged and repeated exposure to blue light emitted by electronic devices. The term "blue light" refers to the high-energy visible (HEV) light spectrum, which is present in the blue region of the visible light spectrum. As we are spending more and more time with or in front of screens from computers, tablets, or smartphones, there are increasing concerns about the harmful impact of blue light on skin. When referring to these light sources, we talk about artificial blue light. Dr Ludger Kolbe (Chief Scientist Photobiology) tested the radiation onto the skin emitted by different smartphones and tablets from various distances. Findings: the amount of blue light emitted during the conventional use of electronic devices is by far not enough to trigger harmful skin effects. If you sit in front of a monitor uninterrupted for a week at a distance from the screen of approximately 30 cm, this would be the same as the blue light intensity of spending one minute outside on a sunny day in Hamburg Germany at around midday at midsummer. If you hold a smartphone right next to the skin, the intensity does increase, but it would still take approximately 10 hours of uninterrupted use to match the effect on the skin of just one minute of sunlight as described. The emissions from electronic devices are barely noticeable in comparison to natural blue light directly from the sun and are, thus, negligible. The same does not apply for natural HEVIS, which does harm the skin. So is HEVIS from a screen is not causes screen dermatitis, what does? Overall, there is an overlap with skin complaints in buildings with ‘climate control’ problems (2), which may explain why screen dermatitis is relatively more common in atopic patients. The same authors found psychosocial conditions and high work-related stress to be indicators for developing VDT-related facial skin problems. Berg et al. (3) found that these patients frequently have sensitive skin: they are so-called ‘stingers’, reacting with stinging or itching when lactic acid (5%) (LAST-test or Lactic Acid Sting Test). In a large literature study, screen dermatitis was found to show many similarities with skin damaged by UV light or ionizing radiation (4). Ionizing radiation consisting of particles, rays with sufficient energy to cause ionization in the medium through which it passes. Examples are heat or light from the sun, microwaves from an oven, X rays from an X-ray tube and gamma rays from radioactive elements. In this large literature study, not only clinical but also immuno-histological manifestations were evaluated. Most striking was the increased number of mast cells in screen dermatitis, containing histamine. The latter is known to be released when mast cells are exposed to UV light and may be responsible for symptoms of itch, pain, oedema and erythema in screen dermatitis. Furthermore, Langerhans' cells in the epidermis were significantly decreased or virtually absent in screen dermatitis as well as in skin damaged by UV light or ionizing radiation. On the other hand, levels of some neuropeptides were determined, and although several differences were found with normal skin, no single marker was 100% able to distinguish between healthy skin and screen dermatitis (1). It is unclear whether VDTs leak electric or magnetic fields that affect our cells (4). In keeping with these findings are the conclusions of a case-referent study, stating that screen dermatitis most likely is the result of non-specific or irritant factors in subjects with sensitive skin (2). In conclusion: It is highly recommended to use SPF and protect the skin from natural HEVIS (with for example product containing Licochalcone A). However protection against artificial blue light won't likely prevent or improve screen dermatitis symptoms. Always consult your dermatologist for a proper diagnose and treatment. Take care. References:

It is widely known that skin´s own hyaluron is a precious molecule keeping our skin hydrated as it is a powerful humectant (attracting and binding water), hence giving the skin a natural plumpness and bounce. What many don´t know is that skin´s own hyaluronic acid content needs to be replenished continuously, as it´s half-life is only several hours up to one day 1. It´s degradation is fastened by 2 different pathways: an external influence via free radical activity or physical degradation and an internal pathway via enzymatic or biological degradation by a family of enzymes called hyaluronidase or abbreviated HYAL.
There are 6 different ones identified and HYAL 1 is the most active one. HYAL 1 “cuts” large size hyaluron molecules (the most capable of binding water) into smaller molecules, which are eliminated even faster. One of the strategies to maintain skin´s own hyaluron content is to inhibit the HYAL enzymes, especially HYAL1. Comparing photo-exposed skin to photoprotected skin showed significant increase in the expression of L-HA (low molecular weight HA) which are smaller or broken hyaluronic acid molecules. An increase of degradated hyaluron was associated with a significant expression of HYAL-1 (2)..UV, ROS or free radical activity leads to the activation of hyaluronidase (3,4). You may now wonder how it is possible that hyaluron filler injections can have a lasting effect of several months or even longer than a year. This is because in those injectable gels the hyaluron molecules are stabilized to protect them from the impact of free radicals and HYAL enzymes. Often this is done with chemical crosslinks (BDDE). Manufacturers of those hyaluron injectable gels use different stabilizing or crosslink technologies and different number of crosslinks, which impacts the gels consistency and longevity. They aren´t completely resistant to hyaluronidase, as it can be used to dissolve injected hyaluron. As hyaluron is anyway depleted and replenished every day, this dissolving procedure hardly affects skin´s own hyaluronic acid content. This is a common misconception. 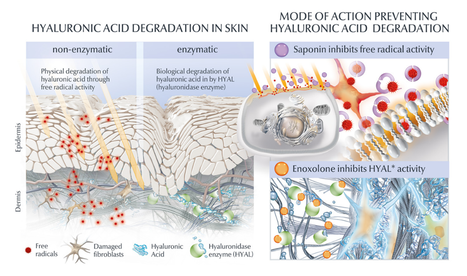
In skin care however, the use of crosslinked hyaluron (hence lasting for months or longer) does not make a lot of sense as we usually cleanse our skin twice daily and thus wash it away. It is too large to penetrate. There are some benefits for crosslinked hyaluron, but it does not impact the longevity of skin´s own hyaluronic acid content. One ingredient derived from the roots of Chinese Licorice plant called Enoxolone (also known as Glycyrrhetinic acid) however, has proven to decrease the HYAL1 activity by 54% (in vitro) (5.6). This is a novel and safe topical way to protect skin´s own hyaluronic acid content from fast degradation and elimination.
However, as mentioned before free radicals increase HYAL1 activity and as we age our skin becomes less resilient against accumulated oxidative stress. Therefore, the most optimal approach to inhibit increasing break down of hyaluronic acid is to combine HYAL1 inhibition with powerful anti-oxidants. In the illustration, which I created professionally, it is Saponin. Saponin is next to a powerful anti-oxidant, also a potent bio-stimulator of the fibroblast for hyaluron (+256%), collagen (+49%) (6) and elastin (+19%). Furthermore, Enoxolone stimulates melanin production, supports the skin's own repair mechanism against UV-induced DNA damage and inhibits enzymatic elastin degradation. What a power-couple to have in dermo-cosmetic products to manage the biological degenerative process of ageing skin. Take care 1. HA: a key molecule in skin aging E. Papakonstantinou 2. Dermatoendocrinol. 2012 Jul 1; 4(3): 253–258. doi: 10.4161/derm.21923 Hyaluronic acid: A key molecule in skin aging Eleni Papakonstantinou, 1 Michael Roth, 2 and George Karakiulakis 1 3. BMC Complementary and Alternative Medicine December 2013, 13:304| In vitro determination of the anti-aging potential of four southern African medicinal plants Authors Gugulethu NdlovuEmail, Gerda Fouche, Malefa Tselanyane, Werner Cordier, Vanessa Steenkamp 4. Bioorg Chem. 2018 Apr;77:159-167. doi: 10.1016/j.bioorg.2017.12.030. Epub 2018 Jan 4. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Madan K1, Nanda S2. 5. EADV Poster 2021 A holistic hyaluron-centric anti-aging concept to improve static and dynamic wrinkles Geloven van A, Harbig S, Stuhr A, Dunckel J, Kuhn A, Dippe R, Warnke K, Beiersdorf AG, Hamburg, Germany 6. EADV Poster 2021 Multifaceted novel approach to increase skin’s own epidermal & dermal hyaluron content Bussmann T, Warnke K, Krüger A, Möller N, Harbig S, Stuhr A, Dunckel J, Geloven van A, Weise J, Beiersdorf AG, Hamburg, Germany 7. Hong et al. Glycyrrhetinic Acid: A Novel Modulator of Human Skin Pigmentation and DNA-Repair September 2009Journal of Investigative Dermatology Conference: 39th Annual European-Society-for-Dermatological-Research Volume: 129 
We all know the fairy tail sleeping beauty, however there is actual scientific evidence supporting that good sleep does make you more attractive. Chronic poor sleep quality is associated with increased signs of intrinsic ageing, diminished skin barrier function, lower satisfaction with appearance (1). A variety of studies in the literature have shown that sleep plays a role in restoring immune system function and that changes in the immune response may affect collagen production. (2) Before going into the science supporting that sleep makes you more beautiful, some tips for better sleep and less wrinkles:
A study was conducted with 60 healthy caucasian women, who were categorised as poor quality sleepers [Pittsburg Sleep Quality Index (PSQI) > 5, sleep duration ≤ 5 h] or good quality sleepers (PSQI ≤ 5, sleep duration 7-9 h. Good sleepers had significantly lower intrinsic skin ageing scores by SCINEXA(TM) . At baseline, poor sleepers had significantly higher levels of TEWL (trans-epidermal water loss). At 72 h after tape stripping, good sleepers had 30% greater barrier recovery compared with poor sleepers. At 24 h after exposure to ultraviolet light, good sleepers had significantly better recovery from erythema (redness). Good sleepers also reported a significantly better perception of their appearance and physical attractiveness compared with poor sleepers. (1) Sleep loss could also impact sexual behaviour and collaboration, because sleep is connected with attractiveness, which has an important impact in many social contexts (2). The notion of beauty sleep is actually very important, because sleep deprived people are perceived as more fatigued, less attractive, and even less healthy than when they are rested (3). Sleep deprivation is associated with increased signs of intrinsic skin ageing (fine lines, uneven pigmentation, reduced elasticity) (4), with much slower recovery rates after skin barrier disruption and lower satisfaction with appearance. If we compare people with a normal night sleep with the same persons but sleep deprived, the sleep deprived ones look more fatigued, with hanging eyelids, redder eyes, more swollen eyes, darker circles under the eyes, paler skin, more wrinkles and fine lines around the eyes, the corners of the mouth as being more droopy and more sad (5). In conclusion, the sleep is not connected only with good health (6,7), but is also a link between attractiveness and health. (2,8) Take care and sleep well

Reading the instructions on cleansing and care products can be misleading. When do I pat my skin dry first or when do I apply the product on damp skin? Even many recommendations from skin care guru's or skinfluencers are not completely correct.
In general it is recommended to apply a serum, eye care or moisturising / hydrating care product on damp skin, or immediately after bathing for the following reasons: Increased Absorption The primary benefit of applying skin care products to damp skin is that the skin is more receptive to the ingredients. Water helps to increase the hydration levels of the skin cells, which then improves the absorption of the skincare products. When the skin is damp, the skin's surface is more permeable, allowing the ingredients in the skin care products to penetrate deeper into the skin, and work their magic. Absorbing the ingredients more effectively, this leads to better results. The exception are products which require a very low pH level to penetrate, and be more effective, for example L-Ascorbic Acid (Vitamin C) and chemical exfoliating "acids" like hydroxy acids. The reason is that water has a pH level of 7-8, acidic formulations will be "neutralised" on damp skin. Better hydration Applying skin care products to damp skin helps to lock in moisture, leaving your skin feeling soft, supple, and hydrated. Hydration is critical for the skin because it helps to maintain and restore the skin's barrier function. The skin barrier protects the skin from losing hydration and prevents irritants and bacteria from entering. Applying serums and moisturisers on damp skin, increases the hydration benefits from the products. Improved spreadability Another advantage of applying skincare on damp skin is that it helps to improve the spreadability of the product. When we apply products such as serum or moisturiser to dry skin, they tend to settle in one area and can be challenging to spread evenly. On the other hand, when applied to damp skin, the skin care products can spread easily and evenly across the skin surface, ensuring maximum coverage and benefit. The exception are lipid rich products which are hydrophobe (water repelling), for example ointments, they might not spread evenly or easy on damp skin. Enhanced performance Applying skin care products to damp skin has been shown to improve their performance. This is because when products are applied to damp skin, they are less likely to evaporate, and the ingredients remain active for longer. This increased contact time with the skin leads to better, more effective results. The exception are products containing vitamin A, retinoids, tretinoin, retinal, retinol, retinaldehyde as damp skin increases the risk of irritation. Sensitive and hyper-sensitive skin Usually people with sensitive and hyper-sensitive skin have an impaired skin barrier function, hence ingredients will penetrate better in comparison to a resilient and well-functioning skin barrier. Applying products on (hyper)sensitive skin will therefore increase the risk of irritations. Be mindful which ingredients you use and use a pH rebalancing toner after cleansing and prior to any serum or care product you use. A toner is anyway an affordable product, which I highly recommend to use in every skin care routine. Read more. Study results on patients with dry skin and healthy volunteers In healthy subjects, compared to at control sites, the Stratum Corneum Water Content (SCW) was significantly higher at sites treated with the moisturizer immediately after bathing, with 1.0 and 2.0 mg/cm2 of the moisturizer, and with once- and twice-daily applications. In patients with dry skin, the SCW was significantly higher compared to control sites after 8 weeks when the moisturizer was applied twice daily. Read more. Take care. 4/30/2023 Comments Skip-care skin care routine
Skip-care is a skincare trend that has taken the beauty world by storm for a while now. It is a Korean beauty inspired technique that focuses on simplifying your skin care routine by skipping steps and using multi-purpose products. This skin minimalistic approach will save time, money and reduces the risk of skin irritation caused by the overuse of certain products or combinations.
Skip-care is rooted in the Korean beauty philosophy of ‘less is more’. The skincare industry in South Korea is one of the most innovative and advanced in the world, and they have been leading the way when it comes to skincare trends. The Korean beauty industry is famous for their 10-step skincare routine, which is aimed at improving overall skin health and beauty. However, this long and high-maintenance routine is not for everyone, and this is where skip-care comes in as an alternative approach. It aims to simplify the routine and accessible to people who do not have the time or resources to devote to a lengthy skincare routine. Skip-care steps:
If you want to go very minimal, you could combine cleanse and tone by using an all-in-one toning cleanser, skip the "treat" step and your skip-care routine is reduced to 2 steps only. Con's:
A benefit on top of saving time, money and reducing the risk of irritation is that by simplifying and streamlining your routine, you probably increase compliance. The best skin care routine is still the one you actually adhere to. Take care 4/30/2023 Comments All you need to know about baby skin
Baby skin is different from adult skin: more sensitive and less resilient. It is important to understand the differences, as it influences how to care for baby skin.
At birth, baby skin undergoes a dramatic change from an aqueous (in the womb) to a dry environment. Baby skin, hair, and fingernails all start to form during the first trimester of pregnancy and continue to develop.
RECOMMENDATIONS FOR BABY SKIN CARE 1. Avoid Fragrance and Other Irritants Fragrances and other irritants can exacerbate skin conditions in babies, such as eczema. Avoid products that contain fragrances, sulfates, and other ingredients that may cause irritation. 2. Use Gentle Cleansers When bathing your baby, use mild, soap-free cleansers that are specifically formulated for baby skin. Avoid using harsh soaps or chemicals that can strip the skin of natural oils. 3. Avoid Over-Bathing While it may be tempting to give your baby frequent baths, it is important to avoid over-bathing. Bathing too frequently can dry out the skin and cause irritation. Aim to bathe your baby no more than every other day, or as recommended by your pediatrician. 4. Moisturise Regularly To help maintain the skin’s natural moisture barrier, apply a gentle, fragrance-free moisturizer to your baby’s skin after bathing. Look for products that are specifically formulated for baby skin, and avoid those that contain ingredients that may cause irritation. 5. Be Sun-Safe Frequent sunburns and exposure to sunlight in childhood are strongly related to melanoma development; therefore, appropriate measures of photoprotection have been considered to decrease the risk of melanoma and nonmelanoma skin cancer. Since baby skin is more susceptible to damage from UV radiation, it is important to protect your baby from the sun. Use a broad-spectrum sunscreen, that is specifically formulated for babies, compliment this with a hat and special UV protective clothes if needed and avoid exposing your baby to direct sunlight during peak hours. 6. Prevent Diaper Rash Protect babies bum 24/7 from a nasty diaper rash. A safe and affordable product known to prevent, soothes and treats diaper rash is Aquaphor, There are special baby formulations available which can also include ingredients like zinc oxide and pathenol and off course are fragrance fee. In case of doubts or concerns don't hesitate to consult your pediatrician or pediatric dermatologist. Take care. 
It was always believed that the moment we are born, is the moment we are exposed to environmental influences. The truth is that there is ample evidence that already during pregnancy the mothers behaviour: smoking or food has a significant impact on how well we age. We know that all skin needs to be protected against UV and HEVIS by using sunscreen, especially in sun exposed areas from birth onwards.
Although you can not start too early taking care of your skin, the right age to start with a well-ageing skin care routine is actually just post-adolescence for 3 reasons. 1. During adolescence most start with their first cleansing and care routines to remove access of sebum, debris and reduce plus prevent break-outs or comedones. Boys may already shave facial hair. So teenagers or young adults are used to a morning- and evening skin care routine which benefits the overall sense of well-being. 2. Most commonly growth stops when puberty ends and this is the moment the degenerative biological process starts, even though there are no visible signs yet. 3. Prevention of pre-mature ageing skin is the most effective and efficient strategy. SKIN NEEDS CARE There is a movement stating that normal unproblematic skin doesn't need care. I strongly disagree. The choice of products at this age depends of course on the skin type, skin condition, skin health, and environment (like weather conditions, pollution), however the morning care should always focus on protecting every skin type, using suncreen (UV + HEVIS protection) and ideally complimented by anti-oxidants to reduce damaging free radical activity, while the evening routine should at least include proper cleansing (to remove dirt and pollutants), which may be followed by product catering to specific needs, like for example sebum regulating, barrier repairing or hydrating ingredients. I would not make a differentiation between darker or lighter skin in terms of photoprotection, as dark skin only has a natural SPF of 13.3 and light skin of 3.4, hence both not enough to prevent sun damage. However, dark skin has a lower amount of ceramides in the statum corneum and is therefore more prone to trans-epidermal water loss. LAZY SKIN? If you are afraid of spoiling your skin and making it "lazy" using skin care for a long time, know that all effects from a dermo-cosmetic product are 100% reversible, thus temporary. This is regulated by law and to enjoy the benefits from skin care, you need to keep using the products. When you stop, your skin will bounce back to it's original state at least after a full regeneration cycle of about 28 days. A few things to avoid are: sun-damage, especially burns, over-exfoliation (damaged skin barrier) and slugging of oily or acne-prone skin (breakouts). Take care. 
Polynucleotides (PNs) are a type of biomolecule that have recently gained traction in the field of skin care and aesthetic treatments. PNs are composed of multiple nucleotides, which are the building blocks of DNA and RNA. These biomolecules have shown promise in improving the appearance and health of the skin through their ability to stimulate cell growth (activate growth factors), tissue regeneration incl. collagen production, wound healing, fibroblast proliferation and have anti-inflammatory properties.
Polynucleotides (PN) are linear polymers composed of many nucleotide units and they play a key role in the storage and transmission of genetic information. There are two types of polynucleotides (aka nucleic acid) found in nature: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). As mentioned, PN are composed of nucleotides, which consist of 3 parts: a nitrogenous base, a phosphate group. and a five-carbon sugar (2'-deoxyribose in DNA or ribose in RNA). The five base nucleotides are adenine, guanine, cytosine, thymine, and uracil. A DNA molecule consists of two long polynucleotide chains composed of four types of nucleotide subunits: adenine, thymine, guanine, and cytosine, while RNA uses adenine, guanine, cytosine and uracil. REGENERATIVE AESTHETICS Regenerative aesthetics is an emerging branch of regenerative medicine with therapies or products aimed at recapturing youthful structure and function using the body's own systems. Examples of such treatments are platelet-rich plasma (PRP), the use of exosomes or polynucleotides. Dr. Kate Goldie explained soft tissue regeneration fundamentals as following: 1. Regeneration of tissue architecture (structure): tissue composition - component abundance, ratio's, position, density and biomechanics/integrity 2. Regeneration of tissue function: signaling, cell function, cellular components (incl. senescence), gene expression and molecular interaction. The 3 treatment pillars of regenerative aesthetics are: cells, biocues and bio-stimulatory scaffolds. Key superficial soft quiescent cells are the fibroblasts and adipose derived stem cells. One of the big advantages of regenerative aesthetics is by using the body's own system, the results are natural and focused on "skin health" (function) and "skin quality" (appearance). POLYNUCLEOTIDES IN REGENERATIVE AESTHETICS Polynucleotides are most often natural, highly purified DNA molecules extracted for example from trout gonads and activate specialised cells called myofibroblasts and adipocytes. PN containing devices act as short time temporary fillers thanks to the viscoelasticity of the long DNA fragments and improve skin well‐being (cell growth) and steady self‐repair (tissue regeneration). Studies support their dermal reactivating properties or their efficacy as “bioreactivating primers” of skin. The final outcome is more natural and in‐depth tissue regeneration and a healthier look of the skin: a more radiant complexion, even skin tone, reduced appearance of fine lines, wrinkles and sagging, faster wound healing, improved pore size and skin thickness, elasticity and hydration. Furthermore, PNs are generally well-tolerated by the skin and have a low risk of adverse effects. Their effectiveness may vary depending on the individual's skin type, age, and overall health. The long-term effects of PNs on the skin are not yet fully understood, and more research is needed to determine their safety and efficacy. There are various brands available which use polynucleotides in their (meso-) injection gels. For example Mastelli Srl, Italy NEWEST® (Polynucleotide and Hyaluronic Acid) for bio-revitalization, BR Pharm HP Cell Vitaran Skin Healers, NUCLEADYN® or Nucleofill®. One brand of (synthetic) polynucleotide-based skin care products is Yuva by Dr. Devgan Scientific Beauty. The Yuva line includes a range of products formulated with PNs, such as the Yuva Serum and the Yuva Enhancer. These products are marketed as being able to provide hydrating, anti-aging, and skin-rejuvenating benefits. THE FUTURE OF POLYNUCLEOTIDES While polynucleotides have many benefits, they also have some drawbacks. One of the primary limitations is their instability in certain environments. This instability can make it difficult to synthesize and manipulate polynucleotides in the lab. Moreover their are limitations, risks and ethical concerns harvesting or using (human identical) PN's, and long-term safety and efficacy data is not conclusive. However, PNs are a promising area of research in the field of skin care and aesthetic treatments and regeneration. We can expect to see further advancements in the development of PNs-based products and treatments. PNs are already used in combination with other biomolecules, such as hyaluronic acid, growth factors and anti-oxidants and used in combination with other treatments. For a personal recommendation on which aesthetic treatment is most suitable to aesthetically regenerate your skin, please consult an experienced board certified dermatologist, plastic surgeon or cosmetic doctor. Take care. 4/23/2023 Comments Mood boosting skin care
Mood-boosting skin care can be defined as a skin care routine that includes products and/or tools and techniques which (may be specifically designed) enhance our mood and mental well-being in addition to improving the overall health and appearance of the skin. Research has shown that skin care is becoming more than just a physical experience, but also a therapeutic and emotional one. The role of mood-boosting skin care in providing self-care and supporting mental well-being has become increasingly important and has profound benefits. ESSENTIAL OILS These mood boosting routines typically incorporate products that have essential oils that help to relax, uplift, and calm the mind, which in turn, affects an individual's mood positively. However, I am not a big fan of incorporating essential oils in a skin care routine as they can be irritating, cause skin sensitivity, redness and breakouts. Irritation and redness can be signs of sub-clinical inflammation and speed up the biological degenerative process called skin ageing or skin inflammageing. It is not a surprise that dermatologists warn against the use of most essential oils on the skin. When it comes to essential oils, it is best to use them in a diffuser and not skin care. Facial oils can be beneficial however in a skin care routine. Click here to read more about the use of facial oils. A SPA "ME" MOMENT The ancient Greeks were the first to suggest that spas and bathing could be used for therapeutic purposes and not just for hygiene and cleanliness (which are basic requirements for good skin health). A warm relaxing bath isn't always good for skin health however (1). On the other side a 20-30 second cold shower after a workout or sauna encourages the release of cold shock proteins. Cold shock proteins may help you maintain your muscle mass when you're too busy to make it to the gym or when you're taking some planned time off your training routine. Some cold shock proteins are known to decrease inflammation and support faster wound healing. Cold water immersion also activates brown fat, tissue that helps keep the body warm and helps it control blood sugar and insulin levels. It helps the body to burn calories, hence to lose weight. Click here to read more. A SPA BONDING MOMENT As time is precious, meaningful time together with friends, your partner or your kids, can boost your sense of satisfaction and strengthen bonds. Funny anecdote was that I was "masking" with my son, who was at the time 12 years old. While relaxing he asked me what his mask was actually doing and I told him that it makes you look younger. He immediately asked me to remove it, scared he would look afterwards like 7 years old. 
JADE ROLLERS & GUA SHA
I love using (refrigerated) jade rollers and gua sha, especially in the under eye area in the morning to reduce puffiness, increase the circulation and lymph drainage. It is important that the tools glide over the skin, don't tug and thus I apply a nourishing care product first. Moreover, applying skin care products using mindful techniques such as massaging in gentle circular motions can be therapeutic, providing a sense of calm and relaxation. SKIN CARE ROUTINES Mindful evening skin care routines have been shown to be particularly beneficial in unwinding after an overwhelming day, helping to reduce stress and induce better sleep. As it turns out, consistent routines provide more stability in your day, which is beneficial for your mental health and stress level regulation. Therewith every morning- and evening skin care routine is mood-boosting. DO GOOD, FEEL BETTER If you treat your self and your skin good, it will make your skin look better and even improve your quality of life. Self-care enhances feelings of self-worth, boost self-esteem and can even give a sense of accomplishment, is thus mood boosting, even without the use of aroma therapy or essential oils. Take care 
Glycation is one of the basic root causes of endogeneous (intrinsic) skin ageing and a very challenging one or almost impossible one to reverse. Glycation is an ageing reaction which begins in early life, developing clinical symptoms at around 30, and progressively accumulates in tissues and skin due to the glycated collagens that are difficult to be decomposed. Glycation occurs naturally in the body when sugars react with proteins and lipids to form advanced glycation end products (AGEs). AGEs can be exogenously ingested (through food consumption), inhaled via tobacco or endogenously produced and formed both intracellularly and extracellularly. AGE modifications lead to dermal stiffening, diminished contractile capacity of dermal fibroblasts, lack of elasticity in the connective tissues, contribute to hyperpigmentation and a yellowish skin appearance. The formation of AGEs is amplified through exogenous factors, e.g., ultraviolet radiation.
AGEs cause changes in the skin through 3 processes:
One study published in the Journal of Investigative Dermatology found that levels of AGEs were higher in the skin of older individuals compared to younger ones. The study also showed that there was a correlation between the level of AGEs and the severity of skin ageing. This suggests that inhibiting the production or accumulation of AGEs in the skin is a potential target for anti-ageing interventions or skin ageing management. AGEs are complex and heterogeneous, more than a dozen AGEs have been detected (however not all) in tissues and can be divided into three categories according to their biochemical properties. AGEs are formed through four pathways:
GLYCATION INHIBITION IS KEY AGEs can be crosslinked through side chains to form a substance of very high molecular weight, which is not easily degraded. The consequences from skin glycation are irreversible. This makes prevention or inhibition of the process the best potential strategy to maintain skin health and ageing skin management. One way to do this is by altering the diet to reduce the intake of sugars and carbohydrates, which are known to contribute to glycation. Several studies have found that reducing sugar intake can result in significant improvements in skin health, including reducing wrinkles and improving skin texture. 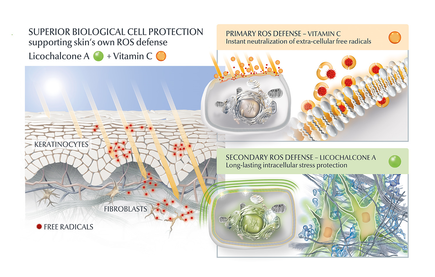
AGE inhibitors
Another potential strategy is the use of topical agents that inhibit the formation or accumulation of AGEs in the skin. One study published in the Journal of Cosmetic Science found that a cream containing carnosine, a peptide that inhibits glycation, improved skin elasticity and reduced the appearance of wrinkles in individuals with ageing skin. Skincare containing NAHP or Acetyl Hydroxyproline inhibits the formation of AGEs significantly (in vitro), most likely through a mechanism where NAHP competes with the proteins for the sugar. Finally, NAHP sacrifices itself in place of the proteins and gets (at least partially) glycated. NAHP also prevents loss of cellular contractile forces in a glycated in vitro dermis model and counteracts the diminished cell-matrix interaction that is caused by glyoxal-induced AGE formation. Anti-Oxidants Moreover, I would suggest to combine those ingredients with an ingredient like Licochalcone A. Numerous high ranked publications support that Licochalcone A protects cells from oxidative stress mediated by e.g. UV and HEVIS (blue light) induced reactive oxidative species (ROS). Due to the activation and nuclear translocation of the transcription factor NrF2, the expression of anti-inflammatory, antioxidant and detoxifying enzymes are induced. These enzymes protect the skin cells (like keratinocytes and fibroblasts) from ROS-induced damage, like lipid peroxidation and DNA as well as protein damage. If Licochalcone A is combined with L-Ascorbic Acid, (the most active form of Vitamin C), it supporting skin's own collagen production, provides superior biological cell protection amongst other relevant benefits. My absolute favourite product is Eucerin Hyaluron-Filler Vitamin C Booster which I use daily as a serum in my morning routine. GLYCATION AND SKIN HEALTH Acne In addition to its role in ageing, glycation in the skin has also been linked to a range of skin health problems. One study published in the Journal of Cosmetic Dermatology found that the level of AGEs in the skin was significantly higher in individuals with acne than in those without acne. The study also showed that treating acne with a topical antibiotic significantly reduced the levels of AGEs in the skin. Atopic Dermatitis Another study published in the Journal of Investigative Dermatology found that individuals with atopic dermatitis had higher levels of AGEs in their skin than healthy individuals. This suggests that glycation may play a role in the development of inflammatory skin conditions. Diabetes + Woundhealing The correlation between high sugar levels and skin ageing can be seen in diabetic patients, where one-third of this population has skin complications. A prominent feature of ageing human skin is the fragmentation of collagen fibers, which severely damages the structural integrity and mechanical properties of the skin. Elevated levels of MMP-1 and MMP-2 and higher crosslinked collagen in the dermis of diabetic skin lead to the accumulation of fragmented and crosslinked collagen, thereby impairing the structural integrity and mechanical properties of dermal collagen in diabetes. Collagen crosslinking makes it impossible for them to easily repair, resulting in reduced skin elasticity and wrinkles. Keratinocytes and fibroblasts are the main cells involved in wound healing, but due to the high glucose (HG) microenvironment in diabetics, the functional state of these cells is impaired, thereby accelerating cellular senescence (programmed cell death). Conclusion We can't completely stop the glycation process, therefore it's important that we inhibit it from a young age onwards, hence monitor the sugar intake of our children, use daily SPF and invest in good dermo-cosmetic products containing ingredients like NAHP and powerful anti-oxidants like L-Ascorbid Acid (Vitamin C is needed for the production of collagen) and Licochalcone A (also anti-inflammatory). Preventing signs of ageing, specifically caused by glycation is most effective. If your skin shows (advanced) signs of ageing, you can get visible improvement using skin component (hyaluron, collagen and elastin) bio-stimulating ingredients like Retinol, Bakuchiol, Arctiin, Creatine or Glycine Saponin. Consult your dermatologist if you wish to improve your skin's appearance or skin health issues. Take care Special thanks: Ph.D. dr Julia M. Weise Manager Biological Testing & Dorothea Schweiger Lab Manager Facial Skin Biology Beiersdorf HQ Hamburg 3/21/2023 Comments Exosomes in skin care and treatments
Skin boosters using micro-injections with predominantly non-crosslinked hyaluron filler gels like Restylane® Vital, Juvéderm® VOLITE or Belotero® Revive are gaining popularity for very good reasons. Unlike traditional dermal fillers, they are not injected beneath the skin to volumise or shape the face. Instead, they are very fine dermal easily integrated "fillers" that are injected into the skin to hydrate, improve skin quality and give very natural results. They are also gently bio-stimulating, meaning they "stretch" the fibroblasts in the injected area and as a result this cell is producing more collagen. An effective bio-remodeling skin booster using 2 times 5 injection points (bio-aesthetic points - BAP) for a full-face treatment is Profhilo®. However, the recent K-beauty treatment via topical application or micro-injections with bio-remodeling exosomes is gaining popularity.
Exosomes are nano-sized cargos with a lipid bilayer structure carrying diverse biomolecules including lipids, proteins, and nucleic acids. These small extra cellular vesicles are secreted by most types of cells (skin relevant are the keratinocytes and fibroblasts) to communicate with each other. Exosomes circulate through bodily fluids and can transfer information. They can be either good or bad, however taken from a healthy young cell they will be sending the best messages. Studies have shown the efficacy of exosomes in skin ageing. They can facilitate skin remodeling (increasing collagen and decreasing senescent cells) leading to skin rejuvenation. Cells sleep because they don't get enough bio-stimulation: messages. Better messages is better skin architecture. This is why exosomes are so important. At the World Stem Cell Summit it used to be 90% about stem cells (they only life 28 days) and 10% about exosomes, now it is 50/50. The reason is called heterochronic parabiosis. 1. One of the most robust methods of improving the function of ageing tissues is that of heterochronic parabiosis,. The effect was shown in a study with a surgical procedure whereby a young and old mouse are joined together so the share one circulatory system. 2 This study according to dr Kate Goldie AMWC 2023 Monaco is proof that it is not the cells, but the messages they give that is transforming lots of different tissues, which has the ability to profoundly regenerate tissues. That is why people are so interested in exosomes. Exosomes taken from a very young cell give potentially the best messages as they "send the message" of youth. EV (Extra-cellular Vesicle) is the actual correct term as messages come as micro-vesicles and exosomes and form 2 different messages from the cell. 3 We start to understand active ingredients. In exosomes one of the most important ingredients is RNA and is part of the future of regenerative aesthetics. Messenger RNAs up-regulate and Micro-RNAs down-regulate. They physically go into the cell and change how the cells works. So we have to be cautious. In this study "The therapeutic and commercial landscape of stem cell vesicles in regenerative dermatology" dr Kate Goldie et al. assessed all available exosomes in the (UK) market. Most exosomes used in-office are extracted from human stem cells and frozen to keep them as stable. Unlike actual stem cells, exosomes don't have a nucleus and therefore they are safe to use. Exosome therapy is the application of topical exosomes after in-office treatments which disrupt the skin barrier, like laser resurfacing, chemical peelings or microneedling. Exosomes are also used in micro-injections as a stand-alone skin boosting treatment and in a few skin care products. Be aware that as usual, not all products are alike. The way exosomes are sourced (origin), size, their content (can be growth factors) and function determine largely their efficacy and the price of the product. One of the challenges is that we do not really know what is in the exosomes. They are like small packages with a lot of messengers. The use of exosomes looks promising for several indications: regenerative aesthetic medicine, healing, scar treatment, burns and atopic dermatitis, however their safety is not yet fully established and no official registration for their use granted. Take care 1. Cell Cycle. 2012 Jun 15; 11(12): 2260–2267. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches Irina M. Conboy 2. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types Methodios Ximerakis 3. J Cell Biol. 2013 Feb 18; 200(4): 373–383. Extracellular vesicles: Exosomes, microvesicles, and friends Graça Raposo et al 3/19/2023 Comments Skin Flooding
Skin flooding is the latest TikTok trend to counteract dry or dehydrated skin. It is basically layering hydrating mists, serums or creams to boost hydration in the skin. The idea is to start with a humectant-rich, lightweight products first and then add a thicker emollient to seal in the moisture on the skin. This is a trend which is suitable for all genders at all ages if products are chosen wisely.
DRY OR DEHYDRATED SKIN There is a difference between dry and dehydrated skin. Dehydrated skin is water-lacking skin, considered a skin condition and can be temporary, while dry skin is lipid-lacking skin and seen as a skin-type. All skin types (yes including oily skin) can be dehydrated. Picture yourself in an environment like airplane without any humidity. Our skin will react to this "dehydration condition" by either (over)production of oils and lipids to "protect" itself fro drying out or get very dehydrated. When the skin is producing a fierce amount of oils and lipids as compensation, you do not have lipid-lacking or dry skin, however your skin might be dehydrated. Lipid-lacking or dry skin has less ability to produce those oils or lipids and often has an impaired skin barrier. Lipids are the "mortar" between the bricks and when they are lacking, more water will evaporate from the skin and thus it loses hydration. FLOODING Therefore "flooding" without "suffocating" the skin, can be a good approach for dehydrated skin and all skin types at all ages. If you want to start your flooding regimen on damp skin, you start immediately after cleansing and toning or use a light mist containing hyaluronic acid and glycerin. Both ingredients are powerful humectants and attract and bind water to the skin surface. Afterwards you might want to first apply a hyaluronic acid containing serum and then a cream to "seal the deal". The difference between flooding and slugging is that flooding is focusing on maximising skin hydration or moisture, while slugging is focusing on prevention of trans-epidermal water loss by (semi)occlusion. Read more about slugging. HYALURONIC ACID Be aware not all hyaluronans are the same. There are different sizes. A macro-hyaluron (about 2000 kDa or larger) will lay on the surface of the skin and bind water there where the skin is losing the most water. A biologicaly active size hyaluron is the 52 kDa micro-hyaluron. This particular size molecule has proven to penetrate into the metabolic active layer of the epidermis, where is actually stimulates the keratinocytes (a certain skin cell) to produce +209% more hyaluron. This can be enough when you are young as the decline of hyaluron in the skin starts first in the epidermis. Be aware it is NOT recommended to use a hyalruon size below 30 kDa in skin care. Hyaluronic acid has the ability to bind and attract water up to 10.000 times is molecular weight, are great to plump up the skin, however the smaller sizes have a different function and can actually harm the skin by for example causing inflammation. LINES AND WRINKLES If you get a bit older, also the dermis will lose it's most important filling substance (hyaluron). There is another skin cell type which can be stimulated to produce more called the fibroblast. This cell is a key cell in the junction of the epidermis and dermis and the dermis, so deeper layer. The powerful anti-oxidant and bio-active Glycine Saponin or abbreviated Saponin can bio-stimulate this cell to produce +256% more skin's own hyaluron, +49% more collagen and +19% more elastin. Moreover, there are products on the market which contain all of the above PLUS Enoxolone. Enoxolone has the ability to inhibit the activity of an enzyme called HYAL1 >50%. HYAL1 is one of 6 different hyaluronidase enzymes which degrate skin's own hyaluron, hence eliminate it from our skin. These enzymes get more active in sun-exposed or mature skin and are partially responsible why our skin will lose hyaluron as we age. Together with the anti-oxidant Saponin, Enoxolone can slow down the elimination of skin's own hyaluron via 2 complimentary pathways: physical and enzymatic degradation. With other words, products containing 2 different sizes of hyaluron, Glycine Saponin and Enoxolone will fill, stimulate and defend skin's own hyaluron in both the epidermis (top layer) and the dermis (middle layer) of the skin. Great for dehydrated and early or visible signs of ageing, like fine lines and (even deepest) wrinkles. DAY AND NIGHT For daytime I would highly recommend a cream with protective SPF and for nighttime a product containing regenerative and barrier repairing Dexpanthenol or Pro-Vitamin B5. Anti-oxidants are good in every day and night time routine. If you want to use a refreshing hydrating mist, there is only one product with hyaluronic acid I recommend and it is Eucerin Hyaluron-Filler Mist Spray. The reason for this is that it has a skin friendly pH unlike (thermal) water. Water has a pH of 7-8 which is like a slight "insult" to the skin's healthy pH every time you spray. With the Hyaluron-Filler Mist Spray, you rebalance the skin's healthy pH level and use it as much as you like. pH to me is the foundation of good skin health. I have written a few pH related blog-posts and if you are interested simply click on the "read more" button below. Take care 3/16/2023 Comments Why slugging should not go viral
One of the current trends in skin care which I don't recommend for most skin types is "slugging". It means that a thick layer of an occlusive or semi-occlusive petrolatum-based product is applied most often shortly prior to bedtime.
One of the benefits of slugging is that this thick layer is acting like an extra barrier for the skin, hence reducing trans-epidermal water loss and penetration of particle matter or irritants. However, for a normal, combination or oily skin with an intact barrier slugging doesn't make any sense and will increase the risk of the development of milia (milk spots) are small, white cysts on your skin especially seen in the under-eye area, comedones (white - or blackheads) and worse papules (inflamed bumps) or pusteles ( a papule with a white or yellow tip). Moreover, a thick layer of product will rub off on your pillow case during the night. Slugging might make sense if your skin barrier is compromised (not intact), for example after a chemical peeling, more invasive laser treatment, over-exfoliation, or when you have extreme dry (lipid lacking) or dehydrated (water lacking) skin. It might also help to prevent irritants or allergens to enter the skin, hence decrease barrier related skin (hyper)sensitivity. However, I would "slug" very consciously and on recommendation of the dermatologist or plastic surgeon after a procedure as there are fantastic products available in the pharmacy or drugstore for (hyper)sensitive, (extreme) dry or dehydrated skin without risking slugging-related skin problems. Instead of a 100% petrolatum-based product, Aquaphor might be a better option at all times (also extreme cold weather), as it doesn't contain water (no risk of freezing), does contain humectants (they can bind and attract water), is semi-occlusive (protects but still "breaths"), is dermatologist recommended, affordable, available in a spray (no touch), tube, or tub and very well researched (evidence based) for a large variety of purposes. Take care |
CategoriesAll Acne Ageing Aquatic Wrinkles Armpits Biostimulators Blue Light & HEVIS Cleansing CoQ10 Cosmetic Intolerance Syndrome Deodorant Dermaplaning Diabetes Dry Skin Evidence Based Skin Care Exfoliation Exosomes Eyes Face Or Feet? Facial Oils Fibroblast Fingertip Units Gendered Ageism Glycation Gua Sha Hair Removal Healthy Skin Heat Shock Proteins Hormesis Humidity Hyaluron Hyaluronidase Hypo-allergenic Indulging Jade Roller Licochalcone A Luxury Skin Care Lymphatic Vessel Ageing Malar Oedema Menopause Mitochondrial Dysfunction Mood Boosting Skin Care Neurocosmetics Ox Inflammageing PH Balance Skin Photo Biomodulation Polynucleotides Psoriasis Regenerative Treatments Review Safety Scarring Sensitive Skin Skin Care Regimen Skin Flooding Skin Hydration Skin Senescence Skip-Care Sleep Slugging Sunscreen Tanning Under Eye Bags Vitamin C Well Ageing Skin Care Wound Healing Wrinkles
Archives
April 2024
|


 RSS Feed
RSS Feed
