
Blue light, is also known as high-energy visible (HEV) light and is the most energetic part of the visible light spectrum (380 - 700 nm) with wavelengths ranging from indigo or ultramarine light 420-440 nanometers, blue light 450-495 nanometers to cyan light 480 - 520 nanometers. Blue light has lower energy than ultraviolet (UV) radiation (280–400 nm) and can reach further into the dermis, up to the depth of 1 mm. [1] Sunlight is the primary natural source of blue light. Up to 50% of the damaging oxidative stress in human skin is generated in the VIS spectrum and the other 50% by UV light [2], contributing to premature ageing, ox-inflammageing and hyperpigmentation like age spots.
Blue light from electronic devices The use of electronic devices has led to increased exposure to artificial blue light sources, however the amount of blue light emitted during the conventional use of electronic devices is by far not enough to trigger harmful skin effects. If you sit in front of a monitor uninterrupted for a week at a distance from the screen of approximately 30 cm, this would be the same as the blue light intensity of spending one minute outside on a sunny day in Hamburg Germany at around midday at midsummer. If you hold a smartphone right next to the skin, the intensity does increase, but it would still take approximately 10 hours of uninterrupted use to match the effect on the skin of just one minute of sunlight. The emissions from electronic devices are barely noticeable in comparison to natural blue light directly from the sun and are, thus negligible. However, blue light or HEV light from sunlight can be harmful for skin. Dr Ludger Kolbe Chief Scientist for Photobiology and his team at Beiersdorf AG did pioneering research regarding the harmful effects of HEVIS. [3-4] I would also like to take the opportunity to debunk an important myth at the start of this article as infrared or near infrared light does not induce damaging free radicals (even in high amounts), there is no such thing "infra-ageing" as a result or IR and in fact red light photobiomodulation supports skin rejuvenation. Read more Direct effects of blue light and HEV Light on skin Blue light and HEV light can have both beneficial and detrimental effects on the skin. The most significant direct effects are mediated through their interaction with chromophores, such as flavins, porphyrins, and opsins, which can trigger the overproduction of reactive oxygen species (ROS), reactive nitrogen species (RNS). and hyperpigmentation. Reactive oxygen and nitrogen species cause DNA damage and modulate the immune response. [1] This oxidative stress can lead to: Photo-ageing: Exposure to blue light and HEV light can induce premature skin aging, causing wrinkles, fine lines, and loss of elasticity. Hyperpigmentation: Blue light and HEV light can stimulate melanin production, leading to uneven skin tone and the development of age spots or other forms of hyperpigmentation. DNA Damage: The ROS and RNS generated by blue light and HEV light can cause DNA damage, plus potentially increase the risk of skin cancer. Inflammation: The oxidative stress triggered by blue light and HEV light can cause an inflammatory response in the skin, exacerbating conditions like acne, eczema, and psoriasis. Molecular and physiological mechanisms of direct blue light effects on the skin [1]
Indirect effects of blue light and HEV Light on skin Blue light and HEV light can also have indirect effects on the skin by disrupting the body's circadian rhythms. This occurs via both the central mechanism, which involves stimulation of light-sensing receptors located in the retina, and via the peripheral mechanism, which involves direct interaction with skin cells. By disrupting the normal circadian rhythm, blue light can negatively affect the skin's natural overnight repair and regeneration processes. [1] The circadian rhythm has been shown to affect multiple cellular and physiological processes occurring in the skin:
Molecular mechanisms of indirect effects of blue light on the skin [1]
Ideal daytime & nighttime skin care regimen When considering cosmetic interventions, a strategy of daytime protection plus defense and night-time repair may be optimal. The skin's own repair mechanisms, such as base excision repair and nucleotide excision repair, attempt to mitigate blue light induced DNA damage. [12] Daytime protection plus defense Of course prevention and/or reduction of blue light exposure from sunlight is key. Reduce the time spent on electronic devices, especially before bedtime, can help minimize the disruption of circadian rhythms and the indirect effects of blue light and HEV light on the skin. Against premature ageing and hyperpigmentation an evidence based effective approach could be the daily use of tinted broadband sunscreen preferably containing Licochalcone A (the most effective anti-oxidant reducing damaging free radical activity from both UV and blue light and moreover protects against collagenase MMP-1 expression) strengthening skin's biological defense [4-5-6-7], while iron oxides in colour pigments provide physical protection against blue light (like zinc oxide and titanium dioxide). Against hyperpigmentation there are (tinted) sunscreens which on top contain the most potent human tyrosinase inhibitor found in dermatological skin care called Thiamidol® [8-9] and one of the 3 ingredients in the "new Kligman Trio" (NT) [18] and Glycyrrhetinic Acid which supports skin's DNA repair and skin pigmentation [10] and inhibits hyaluronidase activity (HYAL1). Most regular sun filters used in sunscreen don't offer any protection against blue light, however according to the website of BASF the chemical UV filters Tinosorb® A2B and Tinosorb® M can reduce the exposure to blue light. [11] Scattering and absorption of blue light [5] The penetration depth of visible light is influenced by the reflection, scattering, and absorption mediated not only by the skin’s physical barrier but also by the VL chromophores in the skin and Fitzpatrick skin or photo-type (FST). The primary VL-scatter and absorption molecules in the skin include hemoglobin, melanin, bilirubin, carotene, lipids, and other structures, including cell nuclei and filamentous proteins like keratin and collagen. Melanin and keratins are the primary VL absorbers and scatterers in the epidermis, while hemoglobin is the dominant absorber, and collagen is the major VL scatter in the dermis. Melanin's absorption spectrum ranges from 200 to 900 nm, with the peak absorption varying based on melanin moiety.. This means that individuals with darker skin types, which have higher melanin content, are more prone to hyperpigmentation from blue light or VIS due to the greater absorption and scattering of VIS in their skin on top of the previously mentioned higher levels of tyrosinase–DCT complexes leading to increased melanogenesis, leading to both transient and long-lasting pigmentation [13], dependent upon the total dose and exacerbation of melasma especially in individuals with FSTs III to VI. Blue light tanning Recent data demonstrate synergistic effects between VL and UV-A on erythema and pigmentation. VL-induced pigmentation is more potent and more sustained than UVA1-induced pigmentation in darker skin tones.Typically, three mechanisms are involved in the responsive reaction of melanocytes to VL, with increased melanin content: immediate pigment darkening (IPD), persistent pigment darkening (PPD), and delayed tanning (DT). [15] Read more. VL can also exacerbate post inflammatory hyperpigmentation (study with FST IV and V). [16] Blue light therapy While the detrimental effects of blue light and HEV light on the skin have been well-documented, these wavelengths have also shown promise in the treatment of certain skin conditions. In controlled clinical settings, blue light has been used to: Treat Acne: Blue light can reduce the growth of Propionibacterium acnes, the bacteria responsible for acne, and has an anti-inflammatory effect. Manage Psoriasis and Atopic Dermatitis: Blue light has been found to have an anti-inflammatory and antiproliferative effect, making it potentially beneficial for the treatment of these chronic inflammatory skin diseases. Reduce Itch: Some studies have suggested that blue light may help alleviate the severity of itching in certain skin conditions. The optimal protocols for blue light therapy are still being developed, and the long-term safety of this treatment modality requires further investigation and should not be initiated without HCP recommendation and monitoring. Vitiligo: Blue light therapy via LEDs can stimulate repigmentation in patients with vitiligo with minimal adverse events, however larger studies are needed. [17] Overall, the research suggests that prolonged or excessive exposure to high-energy blue light, can have negative long-term effects on skin structure, function, and appearance in all phototypes. As our understanding of the individual variations in skin's response to blue light exposure deepens, the development of personalised or tailored effective solutions become increasingly more tangible. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References
Comments

Mitochondria are the "powerhouses" or "lungs" of our cells and bioenergetic semi-autonomous organelles with their own genomes and genetic systems. [1] They are responsible for generating the energy that fuels a wide range of cellular processes in the skin, including cell signaling, pigmentation, wound healing, barrier integrity [2], metabolism and quality control. [3] Mitochondria exist in each cell of the body. Their primary role is cellular respiration; a process converting the energy in nutrients (like glucose) into a usable form of energy called ATP or Adenosine Triphosphate. Mitochondria are particularly abundant in the skin, reflecting the skin's high metabolic demand. When the functionality of mitochondria is impaired or declines, it impacts skin's vitality, health and beauty. Mitochondrial dysfunction is 1 of the 12 hallmarks of skin ageing.
The skin is particularly susceptible to mitochondrial stress due to its constant exposure to environmental insults, such as UV radiation, pollution, and other oxidative stressors. These factors can damage mitochondrial DNA, leading to increased production of reactive oxygen species (ROS) and disrupting the delicate balance of cellular processes. [4] In aged post-mitotic cells, heavily lipofuscin-loaded lysosomes perform poorly, resulting in the enhanced accumulation of defective mitochondria, which in turn produce more reactive oxygen species causing additional damage (the mitochondrial-lysosomal axis theory). [5] Optimal mitochondrial function is indispensable for sustaining the specialized functions of each cell type, like keratinocyte differentiation, fibroblast ECM production, melanocytes melanin production and distribution, immune cell surveillance, sebocytes and adipocytes. [6] Mitochondrial dysfunction is both directly and indirectly linked to chronological ageing and photo-ageing. [7] As mitochondrial function declines, the skin's ability to regenerate and repair itself is decreased. [2=1] This results in visible signs of aging, such as wrinkles, loss of elasticity, dryness, uneven pigmentation, melasma, age spots, lipomas, impaired wound healing. [2-4-5-8-9] Mitochondrial dysfunction also has been implicated in skin conditions like acne, eczema, lupus, psoriasis, vitiligo, atopic dermatitis and even skin cancer. [10] Ageing is associated with changes in mitochondrial morphology, including [6]
Good mitochondrial function or metabolism: [7]
Dysfunctional Mitochondria: [7]
Mitochondrial proteins Mitochondria contain >1,100 different proteins (MitoCoP) that often assemble into complexes and supercomplexes such as respiratory complexes and preprotein translocases. The chaperones Heat Shock Proteins HSP60-HSP10 are the most abundant mitochondrial proteins. [3] Small heat shock proteins form a chaperone system that operates in the mitochondrial intermembrane space. Depletion of small heat shock proteins leads to mitochondrial swelling and reduced respiration. [14] Mitochondrial hyperpigmentation Emerging research has shed light on the intricate relationship between mitochondrial dysfunction and the development of hyperpigmentation, a condition characterized by the overproduction and uneven distribution of melanin in the skin. One of the key mechanisms underlying this connection is the role of mitochondria in the regulation of melanogenesis, the process by which melanin is synthesized. Mitochondria are involved in the production of various cofactors and signaling molecules that are essential for the activity of tyrosinase, the rate-limiting enzyme in melanin synthesis. [15] When mitochondrial function is impaired, it can lead to an imbalance in the production and distribution of these cofactors and signaling molecules, ultimately resulting in the overproduction and uneven deposition of melanin in the skin. [15] This can manifest itself as age spots, melasma, and other forms of hyperpigmentation. The link between mitochondrial dysfunction and hyperpigmentation has been further supported by studies on genetic disorders that involve mitochondrial dysfunction, such as mitochondrial DNA depletion syndrome. In these conditions, patients often exhibit a range of pigmentary skin changes, including patchy hyper- and hypopigmentation, as well as reticular pigmentation. [16] Mitochondrial crosstalk and exosomes Mitochondria can crosstalk and move beyond cell boundaries. [17] Mitochondria-derived material might be transferred to neighboring cells in the form of cell-free mitochondria or included in extracellular vesicles [18-19]. This process supports cellular repair and contributes to vital mitochondrial functions. Besides restoring stressed cells and damaged tissues due to mitochondrial dysfunction, intercellular mitochondrial transfer also occurs under physiological and pathological conditions. [20] The transfer of active mitochondria from mesenchymal stem cells (MSCs) has been identified as a repair mechanism for rejuvenating damaged skin fibroblasts. [21] MITOCHONDRIAL SUPPORT Q10 or Coenzyme Q10 (CoQ10) Q10 is part of the mitochondrial respiration chain and essential for cellular energy production. About 95% of our cellular energy is generated with support of Q10, which is produced by the human body itself. During skin ageing, both the cellular energy production and levels of Q10 are declined. Q10 is a powerful anti-oxidant [22], thus protecting cells from oxidative stress and damage and has proven to be able to "rescue" senescent cells by decreasing elevated senescent markers like p21 levels and β-Galactosidases positive cell numbers (in-vitro). Q10 is bio-active, increasing collagen type I and elastin production. [23=8] Q10 can be supplemented via nutrition, however also via topical application and is considered an evidence based active ingredient in skin care products. Ubiquinol (reduced form) shows higher bioavailability compared to ubiquinone (oxidized form). [23] Glutathione Glutathione is formed in cell's cytoplasm from glutamic acid, cysteine and glycine. It is present in 2 forms: reduced (GSH) and oxidized (GSSG). Reduced GSH is an active anti-oxidant, while the presence of inactive GSSG is increased under oxidative stress. The ratio between GSH and GSSH is considered a measure of oxidative stress. Glutathione participates in redox reactions, acts as co-factor of many anti-oxidant enzymes and is the most important non-enzymatic anti-oxidant, essential for synthesis of proteins and DNA. Low Glutathione results in accelerated ageing and inflammatory skin diseases. Mitochondrial glutathione (mGSH) is the main line of defense for the maintenance of the appropriate mitochondrial redox environment to avoid or repair oxidative modifications leading to mitochondrial dysfunction and cell death. [24] Glutathione can be increased via supplementation via precursors cysteine or N-acetylcysteine (not recommended for pregnant women) or the reduced form of Glutathione itself, or increased via topical active ingredients like Licochalcone A. [25] Nicotinamide NR nicotinamide ribosome which is the precursor of NMN nicotinamide mononucleotide which is the precursor of NAD+ nicotinamide adenine dinucleotide all could have a protective effect on mitochondria. Nicotinamide adenine dinucleotide is present in living organisms as ions NAD+ and NADP+ and in reduced forms NADH and NADPH. NADH is a cofactor of processes inside mitochondria:
Resveratrol Although systemically Resveratrol promotes mitochondrial biogenesis. [27] Other data shows that UVA (14 J/cm(2)) along with resveratrol causes massive oxidative stress in mitochondria. As a consequence of oxidative stress, the mitochondrial membrane potential decreases which results in opening of the mitochondrial pores ultimately leading to apoptosis in human keratinocytes. [28] Red light therapy By incorporating red light therapy into your skin care routine, you can help to counteract the damaging effects of mitochondrial dysfunction and support the skin's natural renewal processes. Next to the use of sunscreens (especially when containing Licochalcone A), CoQ10, anti-oxidants and Nicotinamide, emerging treatments like mitochondrial transfer and therapies focused on improving mitochondrial quality control processes are being investigated as potential solutions for preventing and addressing mitochondrial dysfunction in the skin. As we continue to explore the 12 hallmarks of ageing skin, I am confident that we will gain valuable insights and develop breakthrough innovations that will improve skin quality, health, beauty and vitality. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Many people associate a tan with health, beauty and an active lifestyle. Although a moderate dose of solar radiation is indispensable for our health, unfortunately, there is no such thing as a real "healthy tan" or "healthy sun-kissed glow" as it is always a visible sign of skin damage. Tanning is a response by the skin to exposure to ultraviolet (UV) radiation (and HEV or Blue Light), either from natural sunlight or artificial sources like tanning beds which leads to photo-ageing, pigmentary disorders (like age spots or hyperpigmentation) and immunosuppression, hence skin cancer. When skin is exposed to sunlight: UV rays and high energy visible light (HEV) or also called Blue Light (the most energetic region of HEV), it produces more melanin, a pigment that darkens the skin as a (partial) protective mechanism to prevent further damage. The amount of artificial blue light emitted during the conventional use of electronic devices is not enough to trigger harmful skin effects. (Click here to read more)
MELANIN Melanin is only produced by cells called melanocytes, mostly distributed in the epidermal-dermal junction. Melanocytes contain specialized organelles called melanosomes to store and produce melanin. Melanosomes are transferred from the melanocytes to the neighboring keratinocytes, which are the most abundant cells in the epidermis. One melanin-forming melanocyte surrounded by 36 keratinocytes and a Langerhans cell is called the melano-epidermal unit. [1.2] Melanocytes use the amino acid tyrosine to produce melanin and protect epidermal keratinocytes and dermal fibroblasts from the damaging effects of solar radiation.. [13] The are two melanin pigment classes:
Differences in skin pigmentation do not result from differences in the number of melanocytes in the skin, as one might assume, but from differences in the melanogenic activity (melano-competence), the type of melanin produced in melanosomes (the ratio between eumelanin and pheomelanin differs per Fitzpatrick phototype) and the size, number and packaging of melanosomes, with melanin content of melanosomes ranging from 17.9% to 72.3%. [7] The amount of melanin is never enough for adequate photoprotection, and a "base tan" does not prevent sunburn. Particularly darker phototypes are more sensitive for the damaging effects of Blue Light. Both eumelanin and pheomelanin production are promoted by UV radiation and Blue Light and therefore sunscreens offering a combination of both UV (A + B) protection and Blue Light defense are recommended for all phototypes. TANNING PROCESS The skin's tanning process occurs in four distinct phases: [3]
ROLE OF UVA, UVB AND BLUE LIGHT One of the most important acute effects of UVR is DNA photodamage. UVA and UVB show different properties regarding their biological effects on the skin. [7] Shorter wavelengths (nm) correspond to higher energy. Infrared does not induce oxidative stress. Read more UVA radiation (320-400 nm) penetrates deeper into the skin and can induce indirect DNA damage by the generation of reactive oxygen species (ROS), leading to premature skin aging. UVA, in contrast to UVB is not filtered by window glass, is able to penetrate deeper into the skin and reach the dermis. They are present constantly, with relatively equal intensity, during all daylight hours throughout the year. It has been estimated that 50% of exposure to UVA occurs in the shade. UVA rays are less intense than UVB, but there are 30 to 50 times more of them. To produce the same erythemal response, approximately 1000 times more UVA dose is needed compared with UVB. [7] The bulbs used in tanning beds emit mostly UVA. UVB radiation (280-320 nm) is less prevalent than UVA, primarily affects the outermost layers of the skin, causing direct DNA damage (more potent than UVA) and triggers inflammatory responses that lead to increased melanin production. UVB radiation fluctuates throughout the day, are at their strongest at noon. and are more cytotoxic and mutagenic than UVA. The action spectrum for UV-induced tanning and erythema are almost identical, but UVA is more efficient in inducing tanning whereas UVB is more efficient in inducing erythema (redness). Dark skin is twice as effective compared to light skin in inhibiting UVB radiation penetration. [7] UVB helps the skin to produce Vitamin D. Blue light (400-500 nm) visible light accounts for 50% of sunlight [11] and can contribute to immediate, delayed, continuous and long-lasting pigmentation by activating melanocyte-specific photoreceptors and increasing melanin synthesis, particularly in individuals with darker (melano-competent) skin types [9], cause DNA damage [10] and generate damaging reactive oxygen species in both the epidermis and the dermis. [12] The effects may last longer than those induced by UVA and UVB radiation. Blue Light can penetrate even deeper than UVA and reach the hypodermis. Blue light therapy is used to target acne causing bacteria and inflammation, however the risks might outweigh the benefits especially in darker phototypes and it might worsen acne marks. EPIDERMIS AND DERMIS Both dermal fibroblasts and epidermal keratinocytes play a crucial role in regulating skin pigmentation and tanning response. [13 15] In comparison to epidermal tanning, dermal tanning is less visible, however more immediate. Dermal fibroblasts secrete various paracrine factors that regulate melanocyte function, survival, and melanin production. Factors like hepatocyte growth factor (HGF), nerve growth factor (NGF), stem cell factor (SCF), and basic fibroblast growth factor (bFGF) stimulate melanogenesis and pigmentation [14 15] Fibroblast senescence and altered secretory profiles in conditions like melasma contribute to abnormal pigmentation by stimulating melanogenesis. [15] Epidermal keratinocytes produce factors like α-melanocyte stimulating hormone (α-MSH) and Wnt1 that activate melanogenic pathways in melanocytes, leading to increased melanin synthesis and transfer to keratinocytes. [15 16]. Keratinocyte-derived exosomes can enhance melanin production by melanocytes. [16] Differences in autophagic activity between various keratinocytes also influences pigmentation. [15] Enjoy the sun, however protect your (and your children's) skin from a photo-damaging tan to remain skin health and beauty. Sunless self-tanning products containing dihydroxyacetone (DHA) or Erythrulose provide a safe alternative to achieve a "sun-kissed" glow. You can use after-sun skin care which helps to rehydrate, reduce damage of "sun-stressed" skin and support it's repair. Always consult a qualified healthcare professional or dermatologist to determine what the most suitable approach is for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Many of the skin regenerating or rejuvenating treatments, like energy based devices in the doctors-office are based on the principle to cause controlled damage and therewith provocation of a skin rejuvenating repair response. One of the fascinating mechanisms behind laser "damage" is the heat shock response leading to increased production of regenerating heat shock proteins (HSPs). Heat shock proteins respond to heat stress, are crucial cellular defense mechanisms against stress (environmental and physiological), act as chaperones, aiding in protein folding, prevention of protein damage, cellular protection and repair. [1]
HEAT SHOCK PROTEINS AND OX-INFLAMMAGEING UV radiation and blue light cause oxidative stress and inflammation, and can overwhelm skin's own capacity to counteract the increased formation of reactive oxygen species (ROS) and inflammatory mediators. Chronic oxidative stress state and chronic low grade of inflammation are hallmarks of skin ageing and their combination can be called ox-inflammageing. Oxidative stress and inflammation alter cellular signal transduction pathways and thereby the expression of the ECM genes as well as the structure of the ECM proteins like collagen, fibronectin and elastin. Their reduced expression and increased degradation manifests eventually at the skin surface as wrinkles, loss of firmness, and elasticity. Heat shock proteins are chaperone proteins that facilitate the formation of the ECM and prevention of molecular oxidative damage or degradation and are classified based on their molecular weights.
STIMULATION OF REJUVENATING HEAT SHOCK PROTEINS Heat shock protein synthesis can be initiated not only by heat but also by many chemical and physical stimuli, such as heavy metals, amino acid analogues, oxidative stress, viral infection and UV and ionizing irradiation. [10] Laser Laser treatments have been shown to induce a heat shock response in the skin from epithelial cells to deeper connective tissues, leading to the production of heat shock proteins. This response is characterized by the temporary changes in cellular metabolism, release of growth factors, and increased cell proliferation and thus contribute to tissue regeneration and rejuvenation. [11] CBD It has been proven that a large number of genes belonging to the heat shock protein super-family were up-regulated following cannabidiol (CBD) treatment. [12] UV radiation Ultraviolet radiation (UV)‐induced cell death and sunburn cell formation can be inhibited by previous heat shock exposure and UV itself can induce HSP expression. However, levels of HSP-27 have been found to be elevated in sun‐protected aged skin indicating a link between HSP-27 expression and age‐dependent epidermal alterations. [13] I would recommend daily protection from UV radiation and blue light (or high energy visible light). Ultrasound Ultrasound exposure at different frequencies, intensities, and exposure times can induce HSP-72 expression. Higher ultrasound frequencies, such as 10 MHz, have been found to significantly increase HSP-72 levels. Additionally, increasing the temperature during ultrasound exposure has shown to enhance HSP-72 expression. Interestingly, ultrasound at 1 MHz was unable to induce HSP-72 significantly, while 10 MHz ultrasound induced HSP-72 after 5 minutes of exposure. [10] Radiofrequency Radiofrequency has been shown to increase HSP-70 and decrease melanin synthesis and tyrosinase activity. [14] RF-US treatment significantly increased levels of HSP47 proteins. [15] Red & near infra red light Although I've not seen much peer reviewed published evidence, red light and near infra red light therapy may release the HSPs in the skin if tissue reaches >42 - 45 degrees (even for 8 - 10 seconds). Nicotinamide Nicotinamide and its derivatives have been found to stimulate the expression of heat shock proteins, including HSP-27, HSP-47, HSP-70, and HSP-90 in the skin. These proteins play as mentioned before an essential role in collagen production, skin protection, skin health and rejuvenation. [6] NAD as nutrient interestingly has proven to tweak the epigenome by modulating DNMT1 enzymatic DNA methylation and cell differentiation. [16] In topical applications an ingredient called Dihydromyricetin also called Epicelline® has been successful in inhibiting DNMT1 enzyme activity biochemical assays. [17] Stimulation of heat shock proteins offers a promising and novel invasive, non invasive and topical approach for skin regeneration, rejuvenation and reduction of ox-inflammageing. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References

Like epigenetics and exosomes, neurocosmetics represent a revolutionary approach for skin care incorporating neuroscience principles, leveraging the skin-brain connection to improve skin health and beauty. The term itself is a fusion of the words neuroscience and cosmetics. It differs from psychodermatology which like neurocosmetics connects the interaction between mind and skin, but in a different way. Some describe it as how simple sensory stimulation can improve our overall wellbeing and call it "mood beauty", however this doesn't do it justice as neurocosmetics go beyond mood boosting skincare.
DEFINITION NEUROCOSMETICS Dermatologist Professor Laurent Misery back in 2002 described that neurocosmetics are products which are supposed to modulate the neuro-immuno-cutaneous-system (NICS) function at an epidermal level. Skin cells can produce neuromediators, which are mediators for transmission of information between skin, immune and the nervous system. All skin cells express specific receptors for neuromediators and by binding of the neuromediator to its receptor, modulation of cell properties and skin functions are induced like cell differentiation and proliferation (renewal), pigmentation, etc. Hence, keratinocytes, Langerhans cells, melanocytes, endothelial cells, fibroblasts and the other cells of the skin are modulated and controlled by the nerves and in return skin is able to modulate neuronal activity and growth. [1] SKIN-BRAIN CONNECTION In an article from the International Journal of Novel Research and Developments, the skin-brain connection was described as a psychobiological concept that highlights how emotions, stress, and neurotransmitters impact skin health. Indicating that the skin acts as a neuroimmunoendocrine organ, emphasizing its sensitivity to neural signals and stress responses. [4] CUTANEOUS NERVOUS SYSTEM The skin a sophisticated sensory organ that allows you to interact with your environment through touch and feel. It contains a complex network of nerves that send information about sensations like pressure, pain, itch and temperature from the skin through the spinal cord to the brain [9]. The dynamic interactions between the skin and the nervous system is influenced by factors like stress and inflammation, which can impact skin health and ageing. [7] Nerves in the skin: These nerves are like tiny messengers that tell your brain about what your skin is feeling: pressure, heat or pain. Types of nerve fibers: Some are thick and wrapped in a protective coating, which helps them send messages quickly. Others are thin and slow but are very good at sending messages about pain or temperature changes. [3] Sensory receptors: These receptors can tell if something is touching the skin lightly or if there's a lot of pressure. They can also sense if something is hot, cold, or causing pain. [3] Autonomic nervous system: Part of the cutaneous nervous system helps control things that happen in the skin automatically, like sweating to regulate body temperature. [8] Nerve cells: There are about 20 different types of neurons in our skin. [10] The contribution of epidermal keratinocytes to NICS [3]
CUTANEOUS NEURO-AGEING Neuro-ageing is defined as the changes in the nervous system which cause continuous neurodegeneration due to oxidative stress, neuroinflammation or impaired neuromodulation. As skin ages, Aβ-toxin (increased by oxidative stress) accumulates at the nerve endings innervating the tissue, causing disrupted cellular communication, particularly affecting fibroblasts’ ability to produce collagen and extracellular matrix. On top there is a decrease of nerve growth factor (NGF) production, important for the development and maintenance of nerve cells. Different factors can lead to a drop in NGF production, resulting in malfunctioning keratinocytes and reduced lipolytic activity of adipocytes, visibly impacting skin hydration and firmness. [6] Skin nerve fibres are significantly reduced in number following UV irradiation and in ageing skin [5] and therefore neuro-protectors or targetting neurodegeneration can reduce stress manifestations and promote healthy cellular communication for optimal skin function. [3] Although not much is known regarding skin specific or topical neuroprotectors (most research was focussed on the brain), probably potent anti-oxidants, by significantly reducing oxidative stress from UV and blue light and anti-inflammatory ingredients may inhibit skin neuro-ageing and can be neuroprotective especially when combined with sunscreen and strengthening of the skin barrier. NEUROCOSMETIC VARIETY OF ACTIONS
THE FUTURE OF NEUROCOSMETICS The neurocosmetics market is booming, with a projected value of USD 2.69 billion by 2030. [11] The future of neurocosmetics holds promise for innovative ingredients and concepts that harness new neuroscientific insights to revolutionize skin care and sunscreen formulations, to cater to both physical and emotional aspects of skin health and beauty. Take care! Anne-Marie References

While factors like genetics and lifestyle (including sun exposure) play significant roles in skin ageing, the role of the lymphatic system in skin ageing is an overlooked however interesting strategy to improve skin's youthful functional (health) and physical attributes (beauty).
The lymphatic system, a vital part of the immune system, is responsible for draining excess fluid, toxins, and waste products from tissues. In the skin, lymphatic vessels collect waste and transport it to lymph nodes for filtration. The lymphatic vessels work with tiny, reflexive muscular contractions constantly pumping cleansing (toxins and debris) lymph fluid through their channels. Interestingly it explains why injections with the muscle relaxant botulinum toxin can cause oedema. The function of the lymphatic system
As we age the lymphatic function and density is decreasing 1:
Effects of lymphatic system decline on skin:

Rejuvenating the lymphatic system for youthful sculpted skin:
Wrongful injected fillers in the tear trough or malar (eye socket - cheek area) septum can lead to worsening of malar oedema (fluid retention) or malar bags. Always consult a qualified healthcare professional or dermatologist to determine the most suitable approach for your particular skin condition and rejuvenation goals. Take care! Anne-Marie References: 1. Structural and Functional Changes in Aged Skin Lymphatic Vessels R. Kataru et al. Front. Aging, 2022 2. Reduction of lymphatic vessels in photodamaged human skin Kentaro Kajiya, Rainer Kunstfeld, Michael Detmar, Jin Ho Chung J Dermatol Sci. 2007 3. Patent Cosmetic preparations comprising natural activators 4. Patent Cosmetic preparations comprising daphne extracts 
Glycation is one of the basic root causes of endogeneous (intrinsic) skin ageing and a very challenging one or almost impossible one to reverse. Glycation is an ageing reaction which begins in early life, developing clinical symptoms at around 30, and progressively accumulates in tissues and skin due to the glycated collagens that are difficult to be decomposed. Glycation occurs naturally in the body when sugars react with proteins and lipids to form advanced glycation end products (AGEs). AGEs can be exogenously ingested (through food consumption), inhaled via tobacco or endogenously produced and formed both intracellularly and extracellularly. AGE modifications lead to dermal stiffening, diminished contractile capacity of dermal fibroblasts, lack of elasticity in the connective tissues, contribute to hyperpigmentation and a yellowish skin appearance. The formation of AGEs is amplified through exogenous factors, e.g., ultraviolet radiation.
AGEs cause changes in the skin through 3 processes:
One study published in the Journal of Investigative Dermatology found that levels of AGEs were higher in the skin of older individuals compared to younger ones. The study also showed that there was a correlation between the level of AGEs and the severity of skin ageing. This suggests that inhibiting the production or accumulation of AGEs in the skin is a potential target for anti-ageing interventions or skin ageing management. AGEs are complex and heterogeneous, more than a dozen AGEs have been detected (however not all) in tissues and can be divided into three categories according to their biochemical properties. AGEs are formed through four pathways:
GLYCATION INHIBITION IS KEY AGEs can be crosslinked through side chains to form a substance of very high molecular weight, which is not easily degraded. The consequences from skin glycation are irreversible. This makes prevention or inhibition of the process the best potential strategy to maintain skin health and ageing skin management. One way to do this is by altering the diet to reduce the intake of sugars and carbohydrates, which are known to contribute to glycation. Several studies have found that reducing sugar intake can result in significant improvements in skin health, including reducing wrinkles and improving skin texture. 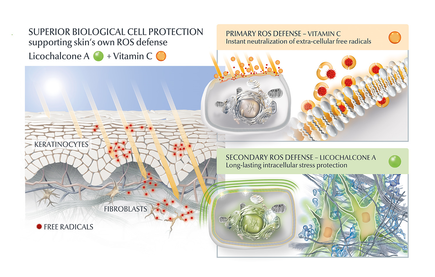
AGE inhibitors
Another potential strategy is the use of topical agents that inhibit the formation or accumulation of AGEs in the skin. One study published in the Journal of Cosmetic Science found that a cream containing carnosine, a peptide that inhibits glycation, improved skin elasticity and reduced the appearance of wrinkles in individuals with ageing skin. Skincare containing NAHP or Acetyl Hydroxyproline inhibits the formation of AGEs significantly (in vitro), most likely through a mechanism where NAHP competes with the proteins for the sugar. Finally, NAHP sacrifices itself in place of the proteins and gets (at least partially) glycated. NAHP also prevents loss of cellular contractile forces in a glycated in vitro dermis model and counteracts the diminished cell-matrix interaction that is caused by glyoxal-induced AGE formation. Anti-Oxidants Moreover, I would suggest to combine those ingredients with an ingredient like Licochalcone A. Numerous high ranked publications support that Licochalcone A protects cells from oxidative stress mediated by e.g. UV and HEVIS (blue light) induced reactive oxidative species (ROS). Due to the activation and nuclear translocation of the transcription factor NrF2, the expression of anti-inflammatory, antioxidant and detoxifying enzymes are induced. These enzymes protect the skin cells (like keratinocytes and fibroblasts) from ROS-induced damage, like lipid peroxidation and DNA as well as protein damage. If Licochalcone A is combined with L-Ascorbic Acid, (the most active form of Vitamin C), it supporting skin's own collagen production, provides superior biological cell protection amongst other relevant benefits. My absolute favourite product is Eucerin Hyaluron-Filler Vitamin C Booster which I use daily as a serum in my morning routine. GLYCATION AND SKIN HEALTH Acne In addition to its role in ageing, glycation in the skin has also been linked to a range of skin health problems. One study published in the Journal of Cosmetic Dermatology found that the level of AGEs in the skin was significantly higher in individuals with acne than in those without acne. The study also showed that treating acne with a topical antibiotic significantly reduced the levels of AGEs in the skin. Atopic Dermatitis Another study published in the Journal of Investigative Dermatology found that individuals with atopic dermatitis had higher levels of AGEs in their skin than healthy individuals. This suggests that glycation may play a role in the development of inflammatory skin conditions. Diabetes + Woundhealing The correlation between high sugar levels and skin ageing can be seen in diabetic patients, where one-third of this population has skin complications. A prominent feature of ageing human skin is the fragmentation of collagen fibers, which severely damages the structural integrity and mechanical properties of the skin. Elevated levels of MMP-1 and MMP-2 and higher crosslinked collagen in the dermis of diabetic skin lead to the accumulation of fragmented and crosslinked collagen, thereby impairing the structural integrity and mechanical properties of dermal collagen in diabetes. Collagen crosslinking makes it impossible for them to easily repair, resulting in reduced skin elasticity and wrinkles. Keratinocytes and fibroblasts are the main cells involved in wound healing, but due to the high glucose (HG) microenvironment in diabetics, the functional state of these cells is impaired, thereby accelerating cellular senescence (programmed cell death). Conclusion We can't completely stop the glycation process, therefore it's important that we inhibit it from a young age onwards, hence monitor the sugar intake of our children, use daily SPF and invest in good dermo-cosmetic products containing ingredients like NAHP and powerful anti-oxidants like L-Ascorbid Acid (Vitamin C is needed for the production of collagen) and Licochalcone A (also anti-inflammatory). Preventing signs of ageing, specifically caused by glycation is most effective. If your skin shows (advanced) signs of ageing, you can get visible improvement using skin component (hyaluron, collagen and elastin) bio-stimulating ingredients like Retinol, Bakuchiol, Arctiin, Creatine or Glycine Saponin. Consult your dermatologist if you wish to improve your skin's appearance or skin health issues. Take care Special thanks: Ph.D. dr Julia M. Weise Manager Biological Testing & Dorothea Schweiger Lab Manager Facial Skin Biology Beiersdorf HQ Hamburg 
There is data suggesting that skin is more prone to sun damage when skin has higher pH levels (>6). The daily use of sunscreen defends the acid mantle by protecting skin cells from sun damage and increasing the skin's ability to protect itself.
Some active ingredients used in sunscreen can increase skin´s optimal pH and are less effective at lowered pH. Ingredients like zinc oxide and titanium oxide (mineral or physical sunscreens) have an optimal “pH window” in which they are more effective. If the pH of zinc oxide or titanium oxide is too low, the oxides will actually dissolve and thus lose efficacy. I understand that some of you might be in favor of only using mineral or physical sunscreen, however with chemical filters or a mix, you most probably maintain a more optimal pH-balance. This is particularly something to consider if you have acne-prone skin, problematic skin, or a sensitive or (very) dry skin type as your skin barrier might already be compromised. Hope you will enjoy the sun well-protected, while maintaining healthy skin. |
CategoriesAll Acne Ageing Aquatic Wrinkles Armpits Biostimulators Blue Light & HEVIS Cleansing CoQ10 Cosmetic Intolerance Syndrome Deodorant Dermaplaning Diabetes Dry Skin Evidence Based Skin Care Exfoliation Exosomes Eyes Face Or Feet? Facial Oils Fibroblast Fingertip Units Gendered Ageism Glycation Gua Sha Hair Removal Healthy Skin Heat Shock Proteins Hormesis Humidity Hyaluron Hyaluronidase Hypo-allergenic Indulging Jade Roller Licochalcone A Luxury Skin Care Lymphatic Vessel Ageing Malar Oedema Menopause Mitochondrial Dysfunction Mood Boosting Skin Care Neurocosmetics Ox Inflammageing PH Balance Skin Photo Biomodulation Polynucleotides Psoriasis Regenerative Treatments Review Safety Scarring Sensitive Skin Skin Care Regimen Skin Flooding Skin Hydration Skin Senescence Skip-Care Sleep Slugging Sunscreen Tanning Under Eye Bags Vitamin C Well Ageing Skin Care Wound Healing Wrinkles
Archives
April 2024
|


 RSS Feed
RSS Feed
